Abstract
Context
The primary treatment of choice for Cushing’s disease (CD) is the removal of the pituitary adenoma by transsphenoidal surgery (TSS). The surgical failure is seen in up to 75% of cases depending on the experience of the surgeon in different studies. Medical therapy is one of the options for the treatment of recurrent or persistent CD.
Methodology
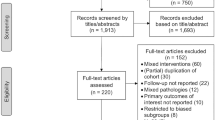
The primary outcome of this meta-analysis was to find the proportion of patients achieving normalisation of 24-h urinary free cortisol (remission of CD) following cabergoline monotherapy. Literature search was conducted in January 2018 in PubMed/MEDLINE database from its date of inception to 31st December 2017. The search strategy used was “[(cushing) OR Cushing’s] AND cabergoline”. Individual participant data were extracted from the included studies and risk of bias was analysed by review checklist proposed by MOOSE.
Results
The individual participant data of 124 patients from six observational studies were included in this meta-analysis. 92 patients (74.2%) had past pituitary surgery. The proportion of patients achieving remission of CD with cabergoline monotherapy was 39.4% (95% confidence interval 0.31–0.49; P = 0.026). The previous surgery [odds ratio (OR) 28.4], duration of cabergoline monotherapy (OR 1.31) and maximum cabergoline dose (OR 0.19) were predictors for remission of CD. Mild and severe side effects were reported in 37.3% and 5.6% of patients, respectively, during cabergoline monotherapy.
Conclusions
This meta-analysis shows that cabergoline monotherapy is a reasonable alternative for subjects with persistent or recurrent CD after TSS. It can also be used in CD patients either as a bridge therapy while waiting for surgery or in those unwilling for surgery or have contraindication to it.


Similar content being viewed by others
Abbreviations
- ACTH:
-
Adrenocorticotropic hormone
- CD:
-
Cushing’s disease
- CI:
-
Confidence interval
- CS:
-
Cushing’s syndrome
- D2R:
-
Type 2 dopamine receptor
- DM:
-
Diabetes mellitus
- DR:
-
Dopamine receptor
- IPD:
-
Individual participant data
- IQR:
-
Interquartile range
- OR:
-
Odds ratio
- SD:
-
Standard deviation
- TSS:
-
Transsphenoidal surgery
- UFC:
-
Urinary free cortisol
- UNL:
-
Upper normal limit
References
Newell-Price J, Bertagna X, Grossman AB, Nieman LK (2006) Cushing’s syndrome. Lancet 367:1605–1617. https://doi.org/10.1016/S0140-6736(06)68699-6
Pivonello R, De Martino MC, De Leo M et al (2008) Cushing’s Syndrome. Endocrinol Metab Clin North Am 37:135–149. https://doi.org/10.1016/j.ecl.2007.10.010
Mancini T, Kola B, Mantero F et al (2004) High cardiovascular risk in patients with Cushing’s syndrome according to 1999 WHO/ISH guidelines. Clin Endocrinol (Oxf) 61:768–777. https://doi.org/10.1111/j.1365-2265.2004.02168.x
Pivonello R, Faggiano A, Lombardi G, Colao A (2005) The metabolic syndrome and cardiovascular risk in Cushing’s syndrome. Endocrinol Metab Clin North Am 34:327–339. https://doi.org/10.1016/j.ecl.2005.01.010
Resmini E, Minuto F, Colao A, Ferone D (2009) Secondary diabetes associated with principal endocrinopathies: the impact of new treatment modalities. Acta Diabetol 46:85–95. https://doi.org/10.1007/s00592-009-0112-9
Carolina Di Somma, Rosario Pivonello, Sandro Loche et al (2002) Severe impairment of bone mass and turnover in Cushing’s disease: comparison between childhood-onset and adulthood-onset disease. Clin Endocrinol (Oxf) 56:153–158. https://doi.org/10.1046/j.0300-0664.2001.01454.doc.x
Feelders RA, Pulgar SJ, Kempel A, Pereira AM (2012) The burden of Cushing’s disease: clinical and health-related quality of life aspects. Eur J Endocrinol 167:311–326. https://doi.org/10.1530/EJE-11-1095
Graversen D, Vestergaard P, Stochholm K et al (2012) Mortality in Cushing’s syndrome: a systematic review and meta-analysis. Eur J Intern Med 23:278–282. https://doi.org/10.1016/j.ejim.2011.10.013
Nieman LK, Biller BMK, Findling JW et al (2015) Treatment of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 100:2807–2831. https://doi.org/10.1210/jc.2015-1818
Pivonello R, De Leo M, Cozzolino A, Colao A (2015) The treatment of Cushing’s disease. Endocr Rev 36:385–486. https://doi.org/10.1210/er.2013-1048
Patil CG, Veeravagu A, Prevedello DM et al (2008) Outcomes after repeat transsphenoidal surgery for recurrent Cushing’s disease. Neurosurgery 63:266–271. https://doi.org/10.1227/01.neu.0000313117.35824.9f
Ambrogio AG, Cavagnini F (2016) Role of “old” pharmacological agents in the treatment of Cushing’s syndrome. J Endocrinol Invest 39:957–965. https://doi.org/10.1007/s40618-016-0462-4
Melmed S, Casanueva FF, Hoffman AR et al (2011) Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96:273–288. https://doi.org/10.1210/jc.2010-1692
Sandret L, Maison P, Chanson P (2011) Place of cabergoline in acromegaly: a meta-analysis. J Clin Endocrinol Metab 96:1327–1335. https://doi.org/10.1210/jc.2010-2443
Delgado-López PD, Pi-Barrio J, Dueñas-Polo MT et al (2018) Recurrent non-functioning pituitary adenomas: a review on the new pathological classification, management guidelines and treatment options. Clin Transl Oncol. https://doi.org/10.1007/s12094-018-1868-6
Pivonello R, Ferone D, de Herder WW et al (2004) Dopamine receptor expression and function in corticotroph pituitary tumors. J Clin Endocrinol Metab 89:2452–2462. https://doi.org/10.1210/jc.2003-030837
Pivonello R, De Martino MC, Cappabianca P et al (2009) The medical treatment of Cushing’s disease: effectiveness of chronic treatment with the dopamine agonist cabergoline in patients unsuccessfully treated by surgery. J Clin Endocrinol Metab 94:223–230. https://doi.org/10.1210/jc.2008-1533
Godbout A, Manavela M, Danilowicz K et al (2010) Cabergoline monotherapy in the long-term treatment of Cushing’s disease. Eur J Endocrinol 163:709–716. https://doi.org/10.1530/EJE-10-0382
Vilar L, Naves LA, Azevedo MF et al (2010) Effectiveness of cabergoline in monotherapy and combined with ketoconazole in the management of Cushing’s disease. Pituitary 13:123–129. https://doi.org/10.1007/s11102-009-0209-8
Barbot M, Albiger N, Ceccato F et al (2014) Combination therapy for Cushing’s disease: effectiveness of two schedules of treatment: should we start with cabergoline or ketoconazole? Pituitary 17:109–117. https://doi.org/10.1007/s11102-013-0475-3
Burman P, Edén-Engström B, Ekman B et al (2016) Limited value of cabergoline in Cushing’s disease: a prospective study of a 6-week treatment in 20 patients. Eur J Endocrinol 174:17–24. https://doi.org/10.1530/EJE-15-0807
Ferriere A, Cortet C, Chanson P et al (2017) Cabergoline for Cushing’s disease: a large retrospective multicenter study. Eur J Endocrinol 176:305–314. https://doi.org/10.1530/EJE-16-0662
Lila AR, Gopal RA, Acharya SV et al (2010) Efficacy of cabergoline in uncured (persistent or recurrent) Cushing disease after pituitary surgical treatment with or without radiotherapy. Endocr Pract 16:968–976. https://doi.org/10.4158/EP10031.OR
Feelders RA, de Bruin C, Pereira AM et al (2010) Pasireotide alone or with cabergoline and ketoconazole in Cushing’s disease. N Engl J Med 362:1846–1848. https://doi.org/10.1056/NEJMc1000094
http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018089806. Accessed 9 Mar 2018
Stewart LA, Clarke M, Rovers M et al (2015) Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD statement. JAMA 313:1657–1665. https://doi.org/10.1001/jama.2015.3656
Stroup DF, Berlin JA, Morton SC et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) Group. JAMA 283:2008–2012
Phan K, Tian DH, Cao C et al (2015) Systematic review and meta-analysis: techniques and a guide for the academic surgeon. Ann Cardiothorac Surg 4:112–122. https://doi.org/10.3978/j.issn.2225-319X.2015.02.04
Elshafie O, Osman A, Aamer F et al (2012) Cushing’s Disease: sustained remission in five cases induced by medical therapy with the dopamine agonist cabergoline. Sultan Qaboos Univ Med J 12:493–497
Geer EB, Shafiq I, Gordon MB et al (2017) Biochemical control during long-term follow-up of 230 adult patients with Cushing disease: a multicenter retrospective study. Endocr Pract 23:962–970. https://doi.org/10.4158/EP171787.OR
Espinosa-de-Los-Monteros AL, Sosa-Eroza E, Espinosa E et al (2017) Long-term outcome of the different treatment alternatives for recurrent and persistent Cushing disease. Endocr Pract 23:759–767. https://doi.org/10.4158/EP171756.OR
Illouz F, Dubois-Ginouves S, Laboureau S et al (2006) Use of cabergoline in persisting Cushing’s disease. Ann Endocrinol 67:353–356
Carroll TB, Javorsky BR, Findling JW (2016) Postsurgical recurrent Cushing disease: clinical benefit of early intervention in patients with normal urinary free cortisol. Endocr Pract 22:1216–1223. https://doi.org/10.4158/EP161380.OR
Broersen LHA, Biermasz NR, van Furth WR et al (2018) Endoscopic vs. microscopic transsphenoidal surgery for Cushing’s disease: a systematic review and meta-analysis. Pituitary. https://doi.org/10.1007/s11102-018-0893-3
Colao A, Petersenn S, Newell-Price J et al (2012) A 12-month Phase 3 study of pasireotide in cushing’s disease. N Engl J Med 366:914–924. https://doi.org/10.1056/NEJMoa1105743
Lacroix A, Gu F, Gallardo W et al (2018) Efficacy and safety of once-monthly pasireotide in Cushing’s disease: a 12 month clinical trial. Lancet Diabetes Endocrinol 6:17–26. https://doi.org/10.1016/S2213-8587(17)30326-1
Castinetti F, Guignat L, Giraud P et al (2014) Ketoconazole in Cushing’s disease: is it worth a try? J Clin Endocrinol Metab 99:1623–1630. https://doi.org/10.1210/jc.2013-3628
Baudry C, Coste J, Khalil RB et al (2012) Efficiency and tolerance of mitotane in Cushing’s disease in 76 patients from a single center. Eur J Endocrinol 167:473–481. https://doi.org/10.1530/EJE-12-0358
Nieman LK, Biller BMK, Findling JW et al (2008) The diagnosis of cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 93:1526–1540. https://doi.org/10.1210/jc.2008-0125
Carrasco CA, Coste J, Guignat L et al (2008) Midnight salivary cortisol determination for assessing the outcome of transsphenoidal surgery in Cushing’s disease. J Clin Endocrinol Metab 93:4728–4734. https://doi.org/10.1210/jc.2008-1171
Colao A, Lombardi G, Annunziato L (2000) Cabergoline. Expert Opin Pharmacother 1:555–574. https://doi.org/10.1517/14656566.1.3.555
Missale C, Nash SR, Robinson SW et al (1998) Dopamine receptors: from structure to function. Physiol Rev 78:189–225. https://doi.org/10.1152/physrev.1998.78.1.189
Lamberts SWJ, Lange SAD, Stefanko SZ (1982) Adrenocorticotropin-secreting pituitary adenomas originate from the anterior or the intermediate lobe in cushing’s disease: differences in the regulation of hormone secretion. J Clin Endocrinol Metab 54:286–291. https://doi.org/10.1210/jcem-54-2-286
de Bruin C, Pereira AM, Feelders RA et al (2009) Coexpression of dopamine and somatostatin receptor subtypes in corticotroph adenomas. J Clin Endocrinol Metab 94:1118–1124. https://doi.org/10.1210/jc.2008-2101
Stefaneanu L, Kovacs K, Horvath E et al (2001) Dopamine D2 receptor gene expression in human adenohypophysial adenomas. Endocrine 14:329–336
van der Pas R, Feelders RA, Gatto F et al (2013) Preoperative normalization of cortisol levels in Cushing’s disease after medical treatment: consequences for somatostatin and dopamine receptor subtype expression and in vitro response to somatostatin analogs and dopamine agonists. J Clin Endocrinol Metab 98:E1880–1890. https://doi.org/10.1210/jc.2013-1987
Dali Yin, Sciji Kondo, Juji Takeuchi, Tatsuo Morimura (1994) Induction of apoptosis in murine ACTH-secreting pituitary adenoma cells by bromocriptine. FEBS Lett 339:73–75. https://doi.org/10.1016/0014-5793(94)80387-0
Pivonello R, Ferone D, de Herder WW et al (2004) Dopamine receptor expression and function in human normal adrenal gland and adrenal tumors. J Clin Endocrinol Metab 89:4493–4502. https://doi.org/10.1210/jc.2003-031746
Schopohl J, Gu F, Rubens R et al (2015) Pasireotide can induce sustained decreases in urinary cortisol and provide clinical benefit in patients with Cushing’s disease: results from an open-ended, open-label extension trial. Pituitary 18:604–612. https://doi.org/10.1007/s11102-014-0618-1
Auriemma RS, Pivonello R, Ferreri L et al (2015) Cabergoline use for pituitary tumors and valvular disorders. Endocrinol Metab Clin North Am 44:89–97. https://doi.org/10.1016/j.ecl.2014.10.007
Acknowledgements
We sincerely thank Dr. A Tabarin, Dr. A Ferriere and Dr. Pia Burman for responding to our queries and sharing their patients’ data with us.
Author information
Authors and Affiliations
Contributions
JS and RP were involved in each of the following points: (1) design, (2) data collection, (3) analysis, and (4) writing manuscript. SS and HD were involved in each of the following points: (1) data collection, (2) quality assessment of articles, (3) analysis, and (4) reviewing manuscript. SSK was involved in each of the following points: (1) design, (2) analysis, (3) quality assessment of studies, and (4) reviewing manuscript. SK was involved in each of the following points: (1) design, (2) data collection, and (3) reviewing manuscript. All the authors approved the final version of this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, informed consent was not necessary.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Palui, R., Sahoo, J., Kamalanathan, S. et al. Effect of cabergoline monotherapy in Cushing’s disease: an individual participant data meta-analysis. J Endocrinol Invest 41, 1445–1455 (2018). https://doi.org/10.1007/s40618-018-0936-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-018-0936-7