Abstract
Background
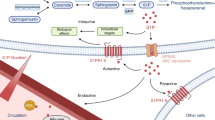
Although recent studies provide clinical evidence that sphingosine-1-phosphate (S1P) may primarily affect bone resorption in humans, rather than bone formation or the osteoclast–osteoblast coupling phenomenon, those studies could not determine which bone resorption mechanism is more important, i.e., chemorepulsion of osteoclast precursors via the blood to bone marrow S1P gradient or receptor activator of NF-κB ligand (RANKL) elevation in osteoblasts via local S1P.
Aim
To investigate how S1P mainly contributes to increased bone resorption in humans, we performed this case–control study at a clinical unit in Korea.
Methods
Blood and bone marrow samples were contemporaneously collected from 70 patients who underwent hip surgery due to either osteoporotic hip fracture (HF) (n = 10) or other causes such as osteoarthritis (n = 60).
Results
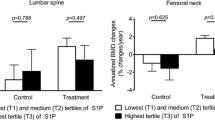
After adjusting for sex, age, BMI, smoking, alcohol, previous fracture, diabetes, and stroke, subjects with osteoporotic HF demonstrated a 3.2-fold higher plasma/bone marrow S1P ratio than those without HF, whereas plasma and bone marrow S1P levels were not significantly different between these groups. Consistently, the risk of osteoporotic HF increased 1.38-fold per increment in the plasma/bone marrow S1P ratio in a multivariate adjustment model. However, the odds ratios for prevalent HF according to the increment in the plasma and bone marrow S1P level were not statistically significant.
Conclusion
Our current results using simultaneously collected blood and bone marrow samples suggest that the detrimental effects of S1P on bone metabolism in humans may depend on the S1P gradient between the peripheral blood and bone marrow cavity.

Similar content being viewed by others
References
Rosen H, Goetzl EJ (2005) Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol 5(7):560–570. doi:10.1038/nri1650
Okajima F, Sato K, Kimura T (2009) Anti-atherogenic actions of high-density lipoprotein through sphingosine 1-phosphate receptors and scavenger receptor class B type I. Endocr J 56(3):317–334
Grey A, Xu X, Hill B, Watson M, Callon K, Reid IR, Cornish J (2004) Osteoblastic cells express phospholipid receptors and phosphatases and proliferate in response to sphingosine-1-phosphate. Calcif Tissue Int 74(6):542–550. doi:10.1007/s00223-003-0155-9
Grey A, Chen Q, Callon K, Xu X, Reid IR, Cornish J (2002) The phospholipids sphingosine-1-phosphate and lysophosphatidic acid prevent apoptosis in osteoblastic cells via a signaling pathway involving G(i) proteins and phosphatidylinositol-3 kinase. Endocrinology 143(12):4755–4763. doi:10.1210/en.2002-220347
Roelofsen T, Akkers R, Beumer W, Apotheker M, Steeghs I, van de Ven J, Gelderblom C, Garritsen A, Dechering K (2008) Sphingosine-1-phosphate acts as a developmental stage specific inhibitor of platelet-derived growth factor-induced chemotaxis of osteoblasts. J Cell Biochem 105(4):1128–1138. doi:10.1002/jcb.21915
Ryu J, Kim HJ, Chang EJ, Huang H, Banno Y, Kim HH (2006) Sphingosine 1-phosphate as a regulator of osteoclast differentiation and osteoclast-osteoblast coupling. EMBO J 25(24):5840–5851. doi:10.1038/sj.emboj.7601430
Ishii M, Kikuta J, Shimazu Y, Meier-Schellersheim M, Germain RN (2010) Chemorepulsion by blood S1P regulates osteoclast precursor mobilization and bone remodeling in vivo. J Exp Med 207(13):2793–2798. doi:10.1084/jem.20101474
Kim BJ, Koh JM, Lee SY, Lee YS, Lee SH, Lim KH, Cho EH, Kim SW, Kim TH, Kim SY, Kim GS (2012) Plasma sphingosine 1-phosphate levels and the risk of vertebral fracture in postmenopausal women. J Clin Endocrinol Metab 97(10):3807–3814. doi:10.1210/jc.2012-2346
Lee SH, Lee SY, Lee YS, Kim BJ, Lim KH, Cho EH, Kim SW, Koh JM, Kim GS (2012) Higher circulating sphingosine 1-phosphate levels are associated with lower bone mineral density and higher bone resorption marker in humans. J Clin Endocrinol Metab 97(8):E1421–E1428. doi:10.1210/jc.2012-1044
Liu H, Chakravarty D, Maceyka M, Milstien S, Spiegel S (2002) Sphingosine kinases: a novel family of lipid kinases. Prog Nucleic Acid Res Mol Biol 71:493–511
Kohama T, Olivera A, Edsall L, Nagiec MM, Dickson R, Spiegel S (1998) Molecular cloning and functional characterization of murine sphingosine kinase. J Biol Chem 273(37):23722–23728
Maeda Y, Seki N, Sato N, Sugahara K, Chiba K (2010) Sphingosine 1-phosphate receptor type 1 regulates egress of mature T cells from mouse bone marrow. Int Immunol 22(6):515–525. doi:10.1093/intimm/dxq036
Peest U, Sensken S-C, Andréani P, Hänel P, Van Veldhoven PP, Gräler MH (2008) S1P-lyase independent clearance of extracellular sphingosine 1-phosphate after dephosphorylation and cellular uptake. J Cell Biochem 104(3):756–772. doi:10.1002/jcb.21665
Zhao Y, Kalari SK, Usatyuk PV, Gorshkova I, He D, Watkins T, Brindley DN, Sun C, Bittman R, Garcia JG, Berdyshev EV, Natarajan V (2007) Intracellular generation of sphingosine 1-phosphate in human lung endothelial cells: role of lipid phosphate phosphatase-1 and sphingosine kinase 1. J Biol Chem 282(19):14165–14177. doi:10.1074/jbc.M701279200
Ishii M, Egen JG, Klauschen F, Meier-Schellersheim M, Saeki Y, Vacher J, Proia RL, Germain RN (2009) Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature 458(7237):524–528. doi:10.1038/nature07713
Ishii M (1831) Kikuta J (2013) Sphingosine-1-phosphate signaling controlling osteoclasts and bone homeostasis. Biochim Biophys Acta 1:223–227. doi:10.1016/j.bbalip.2012.06.002
Dziak R (2013) The role of sphingosine-1-phosphate (S1P) and lysophosphatidic acid (LPA) in regulation of osteoclastic and osteoblastic cells. Immunol Invest 42(7):510–518. doi:10.3109/08820139.2013.823804
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423(6937):337–342. doi:10.1038/nature01658
Ahn SH, Koh JM, Gong EJ, Byun S, Lee SY, Kim BJ, Lee SH, Chang JS, Kim GS (2013) Association of bone marrow sphingosine 1-phosphate levels with osteoporotic hip fractures. J Bone Metab 20(2):61–65. doi:10.11005/jbm.2013.20.2.61
Prevention NIHCDPoO, Diagnosis, and T (2001) OSteoporosis prevention, diagnosis, and therapy. JAMA 285(6):785–795
Siris ES, Miller PD, Barrett-Connor E et al (2001) Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the national osteoporosis risk assessment. JAMA 286(22):2815–2822. doi:10.1001/jama.286.22.2815
Wainwright SA, Marshall LM, Ensrud KE, Cauley JA, Black DM, Hillier TA, Hochberg MC, Vogt MT, Orwoll ES (2005) Hip fracture in women without osteoporosis. J Clin Endocrinol Metab 90(5):2787–2793. doi:10.1210/jc.2004-1568
Sanders KM, Nicholson GC, Watts JJ, Pasco JA, Henry MJ, Kotowicz MA, Seeman E (2006) Half the burden of fragility fractures in the community occur in women without osteoporosis. When is fracture prevention cost-effective? Bone 38(5):694–700. doi:10.1016/j.bone.2005.06.004
Kanis JA, Black D, Cooper C, Dargent P, Dawson-Hughes B, De Laet C, Delmas P, Eisman J, Johnell O, Jonsson B, Melton L, Oden A, Papapoulos S, Pols H, Rizzoli R, Silman A, Tenenhouse A (2002) A new approach to the development of assessment guidelines for osteoporosis. Osteoporos Int 13(7):527–536. doi:10.1007/s001980200069
WHO Study Group (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organization technical report series pp 43:1–129
Parfitt AM, Mathews CH, Villanueva AR, Kleerekoper M, Frame B, Rao DS (1983) Relationships between surface, volume, and thickness of iliac trabecular bone in aging and in osteoporosis. Implications for the microanatomic and cellular mechanisms of bone loss. J Clin Investig 72(4):1396–1409. doi:10.1172/jci111096
Seeman E (2002) Pathogenesis of bone fragility in women and men. Lancet 359(9320):1841–1850. doi:10.1016/s0140-6736(02)08706-8
Borah B, Dufresne TE, Chmielewski PA, Johnson TD, Chines A, Manhart MD (2004) Risedronate preserves bone architecture in postmenopausal women with osteoporosis as measured by three-dimensional microcomputed tomography. Bone 34(4):736–746. doi:10.1016/j.bone.2003.12.013
Acknowledgments
This study was supported by grants from the Korea Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (Project Nos. HI13C1432 and HI14C2185) and the National Research Foundation (NRF) that is funded by the Korean government (Project No. KRF-2010-0025271).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
This study was approved by the Institutional Review Board of Asan Medical Center and was conducted according to the Ethical Principles for Medical Research Involving Human Subjects, as defined by the Declaration of Helsinki.
Informed consent
All enrolled participants provided written informed consent.
Additional information
B.-J. Kim and K.-O. Shin contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, BJ., Shin, KO., Kim, H. et al. The effect of sphingosine-1-phosphate on bone metabolism in humans depends on its plasma/bone marrow gradient. J Endocrinol Invest 39, 297–303 (2016). https://doi.org/10.1007/s40618-015-0364-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-015-0364-x