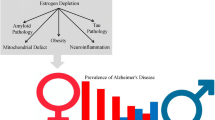
Abstract
Introduction
Experimental and clinical evidence suggests that estrogens have protective effects in the brain. Nevertheless, their potential role against neurodegenerative diseases, in particular Alzheimer’s disease (AD), is still a matter of debate. The identification of the seladin-1 gene (for SELective Alzheimer’s Disease INdicator-1), which appeared to be significantly less expressed in brain region affected in AD, opened a new scenario in the field of neuroprotective mechanisms. Seladin-1 was found to have neuroprotective properties through its anti-apoptotic activity. In addition, it was subsequently demonstrated that seladin-1 also has enzymatic activity, because it catalyzes the conversion of desmosterol into cholesterol. Several studies have shown that an appropriate amount of membrane cholesterol plays a pivotal role to protect nerve cells against β-amyloid toxicity in AD and to counteract the synthesis of β-amyloid.
Methods and Results
We demonstrated that the expression of seladin-1, as well as the synthesis of cell cholesterol, is stimulated by estrogens in human neuronal precursor cells. Cholesterol enriched cells became more resistant against oxidative stress and β-amyloid toxicity. We thus hypothesized that seladin-1 might be a mediator of the neuroprotective effects of estrogens. Indeed, in cells in which seladin-1 gene expression had been silenced by siRNA the protective effects of estrogens were lost. This finding indicates that seladin-1 is a crucial mediator of the neuroprotective effects of these hormones, at least in our cell model.
Conclusions
In summary, these results establish a new link between estrogens and cholesterol, which is represented by the neuroprotective factor seladin-1.



Similar content being viewed by others
References
Greeve I, Hermans-Borgmeyer I, Brellinger C, Kasper D, Gomez-Isla T, Behl C, Levkau B, Nitsch RM (2000) The human DIMINUTO/DWARF1 homolog seladin-1 confers resistance to Alzheimer‘s disease-associated neurodegeneration and oxidative stress. J Neurosci 20:7345–7352
Baulieu EE (1998) Neurosteroids: a novel function of the brain. Psychoneuroendocrinology 23:963–987
Benarroch EE (2007) Neurosteroids: endogenous modulators of neuronal excitability and plasticity. Neurology 68:945–947
Behl C (2003) Estrogen can protect neurons: modes of action. J Steroid Biochem Mol Biol 83:195–197
Maggi A, Ciana P, Belcredito S, Vegeto E (2004) Estrogens in the nervous system: mechanisms and non-reproductive functions. Ann Rev Physiol 66:291–313
Turgeon JL, Carr MC, Maki PM, Mendelsohn ME, Wise P (2006) Complex actions of sex steroids in adipose tissue, the cardiovascular system, and brain: insights from basic science and clinical studies. Endocr Rev 27:576–605
Marron TU, Guerini V, Rusmini P, Sau D, Brevini TAL, Martini L, Poletti A (2005) J Neurochem 92:10–20
Singh M (2006) Endocrine 29:271–274
Veiga S, Melcangi RC, Doncarlos LL, Garcia-Segura LM, Azcoitia I (2004) Sex hormones and brain aging. Exp Gerontol 39:1623–1631
Schumacher M, Guennoun R, Ghoumari A, Massaad C, Robert F, El-Etr M, Akwa Y, Rajkowski K, Baulieu EE (2007) Novel perspectives for progesterone in hormone replacement therapy, with special reference to the nervous system. Endocr Rev 28:387–439
Paganini-Hill A, Henderson VW (1994) Estrogen deficiency and risk of Alzheimer‘s disease in women. Am J Epidemiol 140:256–261
Fillit HM (2002) The role of hormone replacement therapy in the prevention of Alzheimer‘s disease. Arch Intern Med 162:1934–1942
Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J, WHIMS Investigators (2003) Estrogen plus progestin and the incidence of dementia and mild cognitive mechanisms for neuroprotective impairment in post-menopausal women: the women‘s health initiative memory study: a randomized controlled trial. JAMA 289:2651–2662
Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, Gass ML, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D (2003) WHIMS Investigators. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the women’s health initiative memory study a randomized controlled trial. JAMA 289:2663–2672
MacLusky NJ (2004) Estrogen and Alzheimer’s disease: the apolipoprotein connection. Endocrinology 145:3062–3064
Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JC (2002) Cache County Memory Study Investigators, Hormone replacement therapy and incidence of Alzheimer disease in older women. JAMA 288:2123–2129
Henderson VW (2007) Alzheimer’s disease and other neurological disorders. Climacteric 10(Suppl. 2):92–96
O‘Neill K, Chen S, Brinton RD (2004) Impact of the selective estrogen receptor modulator, raloxifene, on neuronal survival and outgrowth following toxic insults associated with aging and Alzheimer’s disease. Exp Neurol 185:63–80
O‘Neill K, Chen S, Brinton RD (2004) Impact of the selective estrogen receptor modulator, tamoxifen, on neuronal outgrowth and survival following toxic insults associated with aging and Alzheimer’s disease. Exp Neurol 188:268–278
Dhandapani KM, Brann DW (2002) Protective effects of estrogen and selective estrogen receptor modulators in the brain. Biol Report 67:1379–1385
Yaffe K, Krueger K, Cummings SR, Blackwell T, Henderson VW, Sarkar S, Ensrud K, Grady D (2005) Effect of raloxifene on prevention of dementia and cognitive impairment in older women: the Multiple Outcome of Raloxifene Evaluation (MORE) randomized trial. Am J Psychiatry 162:683–690
Selkoe DJ (2001) Alzheimer‘s disease: genes, proteins, and therapy. Physiol Rev 81:741–776
Luciani P, Gelmini S, Ferrante E, Lania A, Benvenuti S, Baglioni S, Mantovani G, Cellai I, Ammannati F, Spada A, Serio M, Peri A (2005) Expression of the antiapoptotic gene seladin-1 and octreotide-induced apoptosis in growth hormone-secreting and nonfunctioning pituitary adenomas. J Clin Endocrinol Metab 90:6156–6161
Du B, Ohmichi M, Takahashi K, Kawagoe J, Ohshima C, Igarashi H, Mori-Abe A, Saitoh M, Ohta T, Ohishi A, Doshida M, Tezuka N, Takahashi T, Kurachi H (2004) Both estrogen and raloxifene protect against beta-amyloid-induced neurotoxicity in estrogen receptor alpha-transfected PC12 cells by activation of telomerase activity via Akt cascade. J Endocrinol 183:605–615
Hendriksen PJ, Dits NF, Kokame K, Veldhoven A, van Weerden WM, Bangma CH, Trapman J, Jenster G (2006) Evolution of the androgen receptor pathway during progression of prostate cancer. Cancer Res 66:5012–5020
Biancolella M, Valentini A, Minella D, Vecchione L, D’Amico F, Chillemi G, Gravina P, Bueno S, Prosperini G, Desideri A, Federici G, Bernardini S, Novelli G (2007) Effects of dutasteride on the expression of genes related to androgen metabolism and related pathway in human prostate cancer cell lines. Invest New Drugs 25:491–497
Bonaccorsi L, Luciani P, Nesi G, Mannucci E, Deledda C, Dichiara F, Paglierani M, Rosati F, Masieri L, Serni S, Carini M, Proietti-Pannunzi L, Monti S, Forti G, Danza G, Serio M, Peri A (2008) Androgen receptor regulation of the seladin-1/DHCR24 gene altered expression in prostate cancer. Lab Invest 88:1049–1056
Fuller PJ, Alexiadis M, Jobling T, McNeilage J (2005) Seladin-1/DHCR24 expression in normal ovary, ovarian epithelial and granulosa tumours. Clin Endocrinol (Oxf) 63:111–115
Sarkar D, Imai T, Kambe F, Shibata A, Ohmori S, Siddiq A, Hayasaka S, Funahashi H, Seo H (2001) The human homolog of Diminuto/Dwarf1 gene (hDiminuto): a novel ACTH-responsive gene overexpressed in benign cortisol-producing adrenocortical adenomas. J Clin Endocrinol Metab 86:5130–5137
Luciani P, Ferruzzi P, Arnaldi G, Crescioli C, Benvenuti S, Nesi G, Valeri A, Greeve I, Serio M, Mannelli M, Peri A (2004) Expression of the novel adrenocorticotropic-responsive gene seladin-1/DHCR24 in the normal adrenal cortex and in adrenocortical adenomas and carcinomas. J Clin Endocrinol Metab 89:1332–1339
Battista MC, Roberge C, Otis M, Gallo-Payet N (2007) Seladin-1 expression in rat adrenal gland: effect of adrenocorticotropic hormone treatment. J Endocrinol 192:53–66
Di Stasi D, Vallacchi V, Campi V, Ranzani T, Daniotti M, Chiodini E, Fiorentini S, Greeve I, Prinetti A, Rivoltini L, Pierotti MA, Rodolfo M (2005) DHCR24 gene expression is upregulated in melanoma metastases and associated to resistance to oxidative stress-induced apoptosis. Int J Cancer 115:224–230
Lu X, Kambe F, Cao X, Kozaki Y, Kaji T, Ishii T, Seo H (2008) DHCR24 is a hydrogen peroxide scavenger, protecting cells from oxidative-stress-induced apoptosis. Endocrinology 149:3267–3273
Waterham HR, Koster J, Romeijn GJ, Hennekam RC, Vreken P, Andersson HC, FitzPatrick DR, Kelley RI, Wanders RJ (2001) Mutations in the 3beta-hydroxysterol Delta24-reductase gene cause desmosterolosis, an autosomal recessive disorder of cholesterol biosynthesis. Am J Hum Genet 69:685–694
Kanungo S, Soares N, He M, Steiner RD (2013) Sterol metabolism disorders and neurodevelopment-an update. Dev Disabil Res Rev 17:197–210
Wechsler A, Brafman A, Shafir M, Heverin M, Gottlieb H, Damari G, Gozlan-Kelner S, Spivak I, Moshkin O, Fridman E, Becker Y, Skaliter R, Einat P, Faerman A, Björkhem I, Feinstein E (2003) Generation of viable cholesterol-free mice. Science 19:2079–2087
Mirza R, Hayasaka S, Takagishi Y, Kambe F, Ohmori S, Maki K, Yamamoto M, Murakami K, Kaji T, Zadworny D, Murata Y, Seo H (2006) DHCR24 gene knockout mice demonstrate lethal dermopathy with differentiation and maturation defects in the epidermis. J Invest Dermatol 126:638–647
Mirza R, Hayasaka S, Kambe F, Maki K, Kaji T, Murata Y, Seo H (2008) Increased expression of aquaporin-3 in the epidermis of DHCR24 knockout mice. Br J Dermatol 158:679–684
Yanagisawa K (2002) Cholesterol and pathological processes in Alzheimer’s disease. J Neurosc Res 70:361–366
Reiss AB, Siller KA, Rahman MM, Siller KA, Rahman MM, Chan ES, Ghiso J, De Leon MJ (2004) Cholesterol in neurologic disorders of the elderly: stroke and Alzheimer’s disease. Neurobiol Aging 25:977–989
Björkhem I, Meaney S (2004) Brain cholesterol: long secret life behind a barrier. Arterioscler Thromb Vasc Biol 24:806–815
Arispe N, Doh M (2002) Plasma membrane cholesterol controls the cytotoxicity of Alzheimer’s disease AbetaP (1-40) and (1-42) peptides. FASEB J 16:1526–1536
Yao JK, Wengenack TM, Curran GL, Poduslo JF (2009) Reduced membrane lipids in the cortex of Alzheimer‘s disease transgenic mice. Neurochem Res 34:102–108
Cecchi C, Rosati F, Pensalfini A, Formigli L, Nosi D, Liguri G, Dichiara F, Morello M, Danza G, Pieraccini G, Peri A, Serio M, Stefani M (2008) Seladin-1/DHCR24 protects neuroblastoma cells against abeta toxicity by increasing membrane cholesterol content. J Cell Mol Med 12:1990–2002
Crameri A, Biondi E, Kuehnle K, Lütjohann D, Thelen KM, Perga S, Dotti CG, Nitsch RM, Ledesma MD, Mohajeri MH (2006) The role of seladin-1/DHCR24 in cholesterol biosynthesis, APP processing and Abeta generation in vivo. EMBO J 25:432–443
Abad-Rodriguez J, Ledesma MD, Craessaerts K, Perga S, Medina M, Delacourte A, Dingwall C, De Strooper B, Dotti C (2004) Neuronal membrane cholesterol loss enhances amyloid peptide generation. J Cell Biol 167:953–960
Kaether C, Haass C (2004) A lipid boundary separates APP and secretases and limits amyloid beta-peptide generation. J Cell Biol 167:809–812
Benvenuti S, Luciani P, Vannelli GB, Gelmini S, Franceschi E, Serio M, Peri A (2005) Estrogen and SERMs exert neuroprotective effects and stimulate the expression of seladin-1, a recently discovered anti-apoptotic gene, in human neuroblast long-term cell cultures. J Clin Endocrinol Metab 90:1775–1782
Vannelli GB, Ensoli F, Zonefrati R, Kubota Y, Arcangeli A, Becchetti A, Barni Camici G, Thiele CJ, Balboni GC (1995) Neuroblast long-term cell cultures from human fetal olfactory epithelium respond to odors. J Neurosci 15:4282–4294
Barni T, Maggi M, Fantoni G, Granchi S, Mancina R, Gulisano M, Marra F, Macorsini E, Luconi M, Rotella C, Serio M, Balboni GC, Vannelli GB (1999) Sex steroids and odorants modulate gonadotropin-releasing hormone secretion in primary cultures of human olfactory cells. J Clin Endocrinol Metab 84:4266–4273
Luciani P, Deledda C, Rosati F, Benvenuti S, Cellai I, Dichiara F, Morello M, Vannelli GB, Danza G, Serio M, Peri A (2008) Seladin-1 is a fundamental mediator of the neuroprotective effects of estrogen in human neuroblast long-term cell cultures. Endocrinology 149:4256–4266
Patisaul HB (2005) Phytoestrogen action in the adult and developing brain. J Neuroendocrinol 17:57–64
Setchell KD (2001) Soy isoflavones-benefits and risks from nature’s selective estrogen receptor modulators (SERMs). J Am Coll Nutr 20:354S–362S
Russo VC, Gluckman PD, Feldman EL, Werther GA (2005) The insulin- like growth factor system and its pleiotropic functions in brain. Endocr Rev 26:916–943
Mendez P, Azcoitia I, Garcia-Segura LM (2005) Interdependence of oestrogen and insulin-like growth factor-I in the brain: potential for analyzing neuroprotective mechanisms. J Endocrinol 185:11–17
Zou CG, Cao XZ, Zhao YS, Gao SY, Li SD, Liu XY, Zhang Y, Zhang KQ (2009) The molecular mechanism of endoplasmic reticulum stressinduced apoptosis in PC-12 neuronal cells: the protective effect of insulin-like growth factor I. Endocrinology 150:277–285
Guan J, Gluckman PD (2009) IGF-1 derived small neuropeptides and analogues: a novel strategy for the development of pharmaceuticals for neurological conditions. Br J Pharmacol 157:881–891
Piconi L, Quagliaro L, Assaloni R, Da Ros R, Maier A, Zuodar G, Ceriello A (2006) Constant and intermittent high glucose enhances endothelial cells apoptosis through mitochondrial superoxide overproduction. Diabetes/Metabolism Research and Reviews 22:198–203
Garcia-Segura LM, Arévalo MA, Azcoitia I (2010) Interactions of estradiol and insulin-like growth factor-I signalling in the nervous system: new advances. Prog Brain Res 181:251–272
Znamensky V, Akama KT, McEwen BS, Milner TA (2003) Estrogen levels regulate the subcellular distribution of phosphorylated Akt in hippocampal CA1 dendrites. J Neurosci 23:2340–2347
Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME (1997) Akt phosphorylation of BAD couples survival signals to cell-intrinsic machinery Cell 91:231–241
Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME (1997) Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 1:661–665
Ma ZQ, Santagati S, Patrone C, Pollio G, Vegeto E, Maggi A (1994) Insulin-like growth factors activate estrogen receptor to control the growth and differentiation of the human neuroblastoma cell line SK-ER3. Mol Endocrinol 8:910–918
Patrone C, Gianazza E, Santagati S, Agrati P, Maggi A (1998) Divergent pathways regulate ligand-independent activation of ER alpha in SK-N-BE neuroblastoma and COS-1 renal carcinoma cells. Mol Endocrinol 12:835–841
Font de Mora J, Brown M (2000) IB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol Cell Biol 20:5041–5047
Frago LM, Pañeda C, Dickson SL, Hewson AK, Argente J, Chowen JA (2002) Growth hormone (GH) and GH-releasing peptide-6 increase brain insulin-like growth factor-I expression and activate intracellular signaling pathways involved in neuroprotection. Endocrinology 143:4113–4122
Mendez P, Garcia-Segura LM (2006) Phosphatidylinositol 3 kinase (PI3 K) and glycogen synthase kinase 3 (GSK3) regulate estrogen receptor-mediated transcription in neuronal cells. Endocrinology 147:3027–3039
Giannini S, Benvenuti S, Luciani P, Manuelli C, Cellai I, Deledda C, PezzatiniA Vannelli GB, Maneschi E, Rotella CM, Serio M, Peri A (2008) Intermittent high glucose concentrations reduce neuronal precursor survival by altering the IGF system: the involvement of the neuroprotective factor DHCR24 (Seladin-1). J Endocrinol 198:523–532
Luciani P, Deledda C, Benvenuti S, Cellai I, Modi G, Fibbi B, Danza G, Vannelli GB, Peri A (2012) Relationship between the neuroprotective effects of insulin-like growth factor-1 and 17β-oestradiol in human neuroblasts. J Neuroendocrinol 24:1304–1310
Reyland ME, Evans RM, White EK (2000) Lipoproteins regulate expression of the steroidogenic acute regulatory protein (StAR) in mouse adrenocortical cells. J Biol Chem 275:36637–36644
Ning Y, Chen S, Li X, Ma Y, Zhao F, Yin L (2006) Cholesterol, LDL and 25-hydroxycholesterol regulate expression of the steroidogenic acute regulatory protein in microvascular endothelial cell line (bEnd3). Biochem Biophys Res Commun 342:1249–1256
Acknowledgments
The studies reported in this review were supported by grants from Ente Cassa di Risparmio di Firenze, from the Regione Toscana-Bando Salute 2009 and from Ministero dell’Istruzione, dell’Università e della Ricerca (PRIN 2009 n. 2009YJTBAZ). I wish to thank my collaborators, in particular Drs. Susanna Benvenuti, Paola Luciani, Cristiana Deledda, Corinna Giuliani and Benedetta Fibbi. Without their constant help I would not have been able to write this review. The prize of the Italian Society of Endocrinology is largely “their prize”. I specially thank my family, my wife Silvana together with Niccolò and Giulia, for having supported me throughout all these years. I have to remember my colleagues at the University of Florence, in particular my mentor, Prof. Mario Serio, who always encouraged my ideas and my work. Finally, I wish to mention Prof. Aldo Pinchera. It is a great honor for me to have received a prize entitled to his memory.
Conflict of interest
I declare that I do not have any conflict of interest related to the submitted manuscript.
Ethical approval
I declare that no humans or animals were used by the author and his collaborators to generate the data reported in this review.
Informed consent
No Informed consent was necessary for the data reported in this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peri, A. Neuroprotective effects of estrogens: the role of cholesterol. J Endocrinol Invest 39, 11–18 (2016). https://doi.org/10.1007/s40618-015-0332-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-015-0332-5