Abstract
Objective
Hypoparathyroidism and hypocalcemia are two of the most frequent clinical characteristics of 22q11-deletion syndrome (22q11DS). The aim of this study was to evaluate bone metabolism and density in a cohort of patients affected by 22q11DS.
Methods
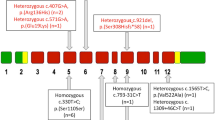
In 8 pediatric patients (mean age 11.5 years; range 7–16.4) affected by 22q11DS, creatinine, albumin, total and ionized calcium, phosphate, 25(OH) vitamin D, parathyroid hormone, osteocalcin, C-terminal telopeptide and interleukin 6 were assessed. Furthermore, bone mineral density (BMD) was determined by dual-energy X-ray absorptiometry procedure. 14 healthy children were considered as controls.
Results
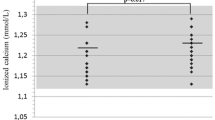
Most of the studied subjects were overweight and lacked quality physical activity. 40 % of the subjects had reduced calcium levels in the absence of related clinical symptoms and all patients also had inadequate levels of Vitamin D. The values of L1-L4 BMD were within the reference range in all patients (z score <2). However, after comparing the age-matched indexes of bone mineralization of patients with those of controls, the former had lower bone mineralization indexes than the latter.
Conclusions
In pediatric patients with 22q11DS, an initial and slight bone loss is evident. The incidence of hypocalcemia is underestimated because hypocalcemia is asymptomatic. Several factors contribute to bone impairment in children who still have to achieve bone mass peak. Therefore, we suggest strict monitoring of bone metabolism as well as BMD measurement in patients affected by 22q11DS.
Similar content being viewed by others
Abbreviations
- 22q11DS:
-
22q11-Deletion syndrome
- BMD:
-
Bone mineral density
- BMI:
-
Body mass index
- PTH:
-
Parathyroid hormone
- CTX:
-
C-terminal telopeptide of type I collagen
- IL-6:
-
Interleukin 6
- BMC:
-
Bone mineral content
- DXA:
-
Dual-energy X-ray absorptiometry
- aBMD:
-
Areal BMD
References
Scambler PJ (2000) The 22q11 deletion syndromes. Hum Mol Genet 9:2421–2426
Oskarsdottir S, Vujic M, Fast A (2004) Incidence and prevalence of the 22q11 delection syndrome: a population-based study in Western Sweden. Arch Dis Child 89:148–151
Bonnet D (2006) Epidemiology and genetics of congenital heart diseases and cardiomyopathies in children. Rev Prat 56:599–604
Robin N, Shprintzen R (2005) Defining the clinical spectrum of delection 22q11.2. J Pediatr 147:90–96
Brauner R, Harivel Le, de Gonneville A (2003) Parathyroid function and growth in 22q11.2 deletion syndrome. J Pediatr 142:504–508
Ruiz JC, Mandel C, Garabedian M (1995) Influence of spontaneous calcium intake and physical exercise on the vertebral and femoral bone mineral density of children and adolescents. J Bone Miner Res 10:675–682
Schoenau E, Land C, Stabrey A, Remer T, Kroke A (2004) The bone mass concept: problems in short stature. Eur J Endocrinol 151(Suppl 1):S87–S91
Stagi S, Lapi E, Gambineri E, Manoni C, Genuardi M, Colarusso G, Conti C, Chiarelli F, de Martino M, Azzari C (2010) Bone density and metabolism in subjects with microdeletion of chromosome 22q11. Eur J Endocrinol 163:329–337
Bassett AS, McDonald-McGinn DM, Devriendt K, Digilio MC, Goldenberg P, V A, Marino B, Oskarsdottir S, Philip N, Sullivan K, Swillen A, Vorstman J, International 22q11.2 Deletion Syndrome Consortium (2011) Pratical guidelines for managing patients with 22q11.2 deletion syndrome. J Pediatr 159:332–339
Nguyen TV, Maynard LM, Towne B, Roche AF, Wisemandle W, Li J, Guo SS, Chumlea WC, Siervogel RM (2001) Sex differences in bone mass acquisition during growth. J Clin Densitom 4:147–157
Weinzimer SA (2001) Endocrine aspects of 22q11.2 deletion syndrome. Genet Med 3:19–22
Cuneo BF, Driscoll DA, Gidding SS, Langman CB (1997) Evolution of latent hypoparathyroidism in familial 22q11 deletion syndrome. Am J Med Genet 69:50–55
Karlsson MK, Linden C, Karlsson C, Johnell O, Obrant K, Seeman E (2000) Exercise during growth and bone mineral density and fractures in old age. Lancet 355:469–470
Karlsson KM, Karlsson C, Ahlborg HG, Valdimarsson O, Ljunghall S, Obrant KJ (2003) Bone turnover responses to changed phisycal activity. Calcif Tissue Int 72:675–680
Peruzzi B, Cappariello A, Del Fattore A, Rucci N, De Benedetti F, Teti A (2012) c-Src and IL-6 inhibit osteoblast differentiation and integrate IGFBP5 signalling. Nat Commun 3:630
Bachrach LK (2001) Acquisition of optimal bone mass in childhood and adolescence. Trends Endocrinol Metab 12:22–28
Mora S, Gilsanz V (2003) Establishment of peak bone mass. Endocrinol Metab Clin North Am 32:39–63
Conflict of interest
The authors declare that they have no conflict of interest. No external funding, apart from the support of the Polytechnic University of Marche, was available for this study.
Compliance with ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from the parents of participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ficcadenti, A., Zallocco, F., Neri, R. et al. Bone density assessment in a cohort of pediatric patients affected by 22q11DS. J Endocrinol Invest 38, 1093–1098 (2015). https://doi.org/10.1007/s40618-015-0295-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-015-0295-6