Abstract
Introduction
3-betahydroxysterol delta-24-reductase (DHCR24), also called selective Alzheimer’s disease indicator-1, is a crucial enzyme in cholesterol biosynthesis with neuroprotective properties that is downregulated in brain areas affected by Alzheimer’s disease.
Aim
In the present study, we investigated modifications of DHCR24 expression in models of Huntington’s disease (HD), a neurodegenerative disorder caused by a polyglutamine expansion in huntingtin (Htt) protein that induces degeneration of cerebral cortex and striatum as well as lateral hypothalamic abnormality.
Methods
Basal expression of DHCR24 and its modulation after oxidative stress were evaluated in rat striatal precursors cells (ST14A) transfected with wild-type (Htt) or mutant Htt (mHtt) and in brain tissue of an HD mouse model (R6/2).
Results
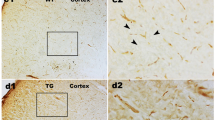
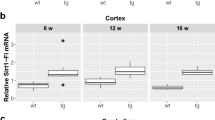
The results showed that DHCR24 transcript levels were decreased in ST14A cells expressing mHtt and in the brain of symptomatic R6/2 mice, but were significantly increased in ST14A cells overexpressing wild-type Htt. In addition, we demonstrated that, in the striatal precursors, the decrease of DHCR24 expression in response to oxidative stress was modified according to the presence of Htt or of its mutant form. Preliminary results indicated a modification of DHCR24 expression in post-mortem brain samples of HD patients.
Conclusions
In conclusion, these results support the hypothesis of a possible role of DHCR24 in HD.





Similar content being viewed by others
Abbreviations
- DHCR24:
-
3-betahydroxysterol delta-24-reductase
- HD:
-
Huntington’s disease
- AD:
-
Alzheimer’s disease
- Htt:
-
Huntingtin
- mHtt:
-
Mutant Htt
- 18S:
-
Ribosomal 18S subunit
References
Waterham H, Koster J, Romeijn G et al (2001) Mutations in the 3beta-hydroxysterol Delta24-reductase gene cause desmosterolosis, an autosomal recessive disorder of cholesterol biosynthesis. Am J Hum Genet 69:685–694
Porter FD (2003) Human malformation syndromes due to inborn errors of cholesterol synthesis. Curr Opin Pediatr 15:607–613
Greeve I, Hermans-Borgmeyer I, Brellinger C et al (2000) The human DIMINUTO/DWARF1 homolog seladin-1 confers resistance to Alzheimer’s disease-associated neurodegeneration and oxidative stress. J Neurosci 20:7345–7352
Crameri A, Biondi E, Kuehnle K et al (2006) The role of seladin-1/DHCR24 in cholesterol biosynthesis, APP processing and Abeta generation in vivo. EMBO J 25:432–443
Cecchi C, Rosati F, Pensalfini A et al (2008) Seladin-1/DHCR24 protects neuroblastoma cells against Abeta toxicity by increasing membrane cholesterol content. J Cell Mol Med 12:1990–2002
Kuehnle K, Crameri A, Kälin R et al (2008) Prosurvival effect of DHCR24/Seladin-1 in acute and chronic responses to oxidative stress. Mol Cell Biol 28:539–550
Peri A, Serio M (2008) Neuroprotective effects of the Alzheimer’s disease-related gene seladin-1. J Mol Endocrinol 41:251–261
Lu X, Kambe F, Cao X et al (2008) 3beta-Hydroxysteroid-delta24 reductase is a hydrogen peroxide scavenger, protecting cells from oxidative stress-induced apoptosis. Endocrinology 149:3267–3273
Peri A, Benvenuti S, Luciani P, Deledda C, Cellai I (2011) Membrane cholesterol as a mediator of the neuroprotective effects of estrogens. Neuroscience 191:107–117
Numakawa T, Matsumoto T, Numakawa Y, Richards M, Yamawaki S, Kunugi H (2011) Protective action of neurotrophic factors and estrogen against oxidative stress-mediated neurodegeneration. J Toxicol 2011:405194
Sharpe LJ, Wong J, Garner B, Halliday GM, Brown AJ (2012) Is seladin-1 really a selective Alzheimer’s disease indicator? J Alzheimers Dis 30:35–39
The Huntington Disease Collaborative Research (1993) G. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 72:971–983
Rigamonti D, Bauer JH, De-Fraja C et al (2000) Wild-type huntingtin protects from apoptosis upstream of caspase-3. J Neurosci 20:3705–3713
Rigamonti D, Sipione S, Goffredo D, Zuccato C, Fossale E, Cattaneo E (2001) Huntingtin’s neuroprotective activity occurs via inhibition of procaspase-9 processing. J Biol Chem 276:14545–14548
Zuccato C, Valenza M, Cattaneo E (2010) Molecular mechanisms and potential therapeutical targets in Huntington’s disease. Physiol Rev 90:905–981
Saleh N, Moutereau S, Durr A et al (2009) Neuroendocrine disturbances in Huntington’s disease. PLoS One 4:e4962
Petersén A, Björkqvist M (2006) Hypothalamic-endocrine aspects in Huntington’s disease. Eur J Neurosci 24:961–967
Browne SE, Beal MF (2006) Oxidative damage in Huntington’s disease pathogenesis. Antioxid Redox Signal 8:2061–2073
Cattaneo E, Rigamonti D, Goffredo D, Zuccato C, Squitieri F, Sipione S (2001) Loss of normal huntingtin function: new developments in Huntington’s disease research. Trends Neurosci 24:182–188
Sipione S, Rigamonti D, Valenza M et al (2002) Early transcriptional profiles in huntingtin-inducible striatal cells by microarray analyses. Hum Mol Genet 11:1953–1965
Valenza M, Leoni V, Tarditi A et al (2007) Progressive dysfunction of the cholesterol biosynthesis pathway in the R6/2 mouse model of Huntington’s disease. Neurobiol Dis 28:133–142
Valenza M, Leoni V, Karasinska JM et al (2010) Cholesterol defect is marked across multiple rodent models of Huntington’s disease and is manifest in astrocytes. J Neurosci 30:10844–10850
Leoni V, Mariotti C, Nanetti L et al (2011) Whole body cholesterol metabolism is impaired in Huntington’s disease. Neurosci Lett 494:245–249
Valenza M, Rigamonti D, Goffredo D et al (2005) Dysfunction of the cholesterol biosynthetic pathway in Huntington’s disease. J Neurosci 25:9932–9939
Leoni V, Mariotti C, Tabrizi SJ et al (2008) Plasma 24S-hydroxycholesterol and caudate MRI in pre-manifest and early Huntington’s disease. Brain 131:2851–2859
Ermak G, Hench KJ, Chang KT, Sachdev S, Davies KJ (2009) Regulator of calcineurin (RCAN1-1L) is deficient in Huntington disease and protective against mutant huntingtin toxicity in vitro. J Biol Chem 284:11845–11853
Mangiarini L, Sathasivam K, Seller M et al (1996) Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87:493–506
Rigamonti D, Bolognini D, Mutti C et al (2007) Loss of huntingtin function complemented by small molecules acting as repressor element 1/neuron restrictive silencer element silencer modulators. J Biol Chem 282:24554–24562
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
DiFiglia M, Sapp E, Chase KO et al (1997) Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 277:1990–1993
Carter RJ, Lione LA, Humby T et al (1999) Characterization of progressive motor deficits in mice transgenic for the human Huntington’s disease mutation. J Neurosci 19:3248–3257
Quinti L, Chopra V, Rotili D, et al. (2010) Evaluation of histone deacetylases as drug targets in Huntington’s disease models. Study of HDACs in brain tissues from R6/2 and CAG140 knock-in HD mouse models and human patients and in a neuronal HD cell model. PLoS Curr 2. doi:10.1371/currents.RRN1172
Bowles KR, Brooks SP, Dunnett SB, Jones L (2012) Gene expression and behaviour in mouse models of HD. Brain Res Bull 88:276–284
Vonsattel JP, Keller C (2008) Del Pilar Amaya M. Neuropathology of Huntington’s disease. Handb Clin Neurol 89:599–618
Kuhn A, Goldstein DR, Hodges A et al (2007) Mutant huntingtin’s effects on striatal gene expression in mice recapitulate changes observed in human Huntington’s disease brain and do not differ with mutant huntingtin length or wild-type huntingtin dosage. Hum Mol Genet 16:1845–1861
Sarchielli E, Marini M, Ambrosini S, et al. (2014) Multifaceted roles of BDNF and FGF2 in human striatal primordium development. An in vitro study. Exp Neurol 257C:130–147
Katsuno M, Adachi H, Sobue G (2009) Getting a handle on Huntington’s disease: the case for cholesterol. Nat Med 15:253–254
Wu C, Miloslavskaya I, Demontis S, Maestro R, Galaktionov K (2004) Regulation of cellular response to oncogenic and oxidative stress by Seladin-1. Nature 432:640–645
McGrath K, Li X, Puranik R et al (2009) Role of 3beta-hydroxysteroid-delta 24 reductase in mediating antiinflammatory effects of high-density lipoproteins in endothelial cells. Arterioscler Thromb Vasc Biol 29:877–882
Otis M, Battista M, Provencher M et al (2008) From integrative signalling to metabolic disorders. J Steroid Biochem Mol Biol 109:224–229
Sarkar D, Imai T, Kambe F et al (2001) The human homolog of Diminuto/Dwarf1 gene (hDiminuto): a novel ACTH-responsive gene overexpressed in benign cortisol-producing adrenocortical adenomas. J Clin Endocrinol Metab 86:5130–5137
Chae JI, Kim DW, Lee N et al (2012) Quantitative proteomic analysis of induced pluripotent stem cells derived from a human Huntington’s disease patient. Biochem J 446:359–371
Illuzzi JL, Vickers CA, Kmiec EB (2011) Modifications of p53 and the DNA damage response in cells expressing mutant form of the protein huntingtin. J Mol Neurosci 45:256–268
Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP (1985) Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol 44:559–577
Jenkins BG, Koroshetz WJ, Beal MF, Rosen BR (1993) Evidence for impairment of energy metabolism in vivo in Huntington’s disease using localized 1H NMR spectroscopy. Neurology 43:2689–2695
Rajkowska G, Selemon LD, Goldman-Rakic PS (1998) Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry 55:215–224
Nieweg K, Schaller H, Pfrieger FW (2009) Marked differences in cholesterol synthesis between neurons and glial cells from postnatal rats. J Neurochem 109:125–134
Acknowledgments
This study was supported by Ministero Istruzione Università e Ricerca (MIUR) (R.M., A.P.), Ente Cassa di Risparmio di Firenze and from Regione Toscana “Bando Salute 2009″, Telethon Foundation (R.M., M.G.). The authors thank Dr.Richard Quinton (Newcastle, UK) and Dr. Francesca Fusco (Rome) for the support in critical reading of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Samara, A., Galbiati, M., Luciani, P. et al. Altered expression of 3-betahydroxysterol delta-24-reductase/selective Alzheimer’s disease indicator-1 gene in Huntington’s disease models. J Endocrinol Invest 37, 729–737 (2014). https://doi.org/10.1007/s40618-014-0098-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-014-0098-1