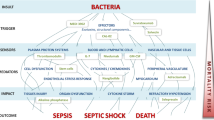
Abstract
Purpose of Review
Sepsis is a clinical condition with a dysregulated immune system majorly attributed to bacteremia. The contrivances underlying this disorder are essential to understand for an early and precise diagnosis. To tackle the burden of challenges like antibiotic resistance and complications of other co-existing metabolic disorders like cancer and diabetes which contribute to an ever-increasing sepsis-related mortality, innovative strategies need to be explored.
Recent Findings
An accurate diagnosis, empirical antibiotic use, and supportive therapy are required for sepsis, preceding which the initial identification of the survival mechanism adopted by the causal pathogen also becomes crucial for strategizing the treatment. In-depth assessments of quinquennial studies available in literature put forth a panel of secreted host-inflammatory cytokines and immune markers that are a prerequisite, to be assessed and then targeted to regulate the host-response mechanism adopted during sepsis. New therapeutic strategies, like drug repurposing for sepsis, immunotherapy, and phytotherapy, can aid in a better management of sepsis; hence, they need to be explored in preclinical and clinical trials. A detailed analysis of lab-based studies on tacking antibiotic-resistant bacteria indicates that phytomolecules can serve as a powerful tool to ameliorate antibiotic resistance and resolve cellular inflammation.
Summary
This review has led us to propose combination therapy as a novel, effective approach whereby potential phytomolecules can be clubbed with available antibiotic arsenal for use as an adjunct in sepsis treatment, for better clinical outcomes.


Similar content being viewed by others
Abbreviations
- ARBs :
-
Antibiotic resistance breakers
- LMICs :
-
Low-middle-income countries
- NK cells :
-
Natural killer cells
- PAMPs :
-
Pathogen-associated molecular patterns
- DAMPs :
-
Damage-associated molecular patterns
- ROS :
-
Reactive oxygen species
- CRP :
-
C-reactive protein
- PCT :
-
Procalcitonin
- AML :
-
Acute myeloid leukemia
- TNF-α :
-
Tumor necrosis factor
- IL :
-
Interleukins
- MDR :
-
Multidrug resistant
- CD :
-
Cluster of differentiation
- NF-κB :
-
Nuclear factor kappa B
References
Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193:259–72.
Schlapbach LJ, Kissoon N, Alhawsawi A, Aljuaid MH, Daniels R, Gorordo-Delsol LA, et al. World Sepsis Day: a global agenda to target a leading cause of morbidity and mortality. Am J Physiol Lung Cell Mol Physiol. 2020;319:518–22.
Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–11.
Santella B, Folliero V, Pirofalo GM, Serretiello E, Zannella C, Moccia G, Santoro E, Sanna G, Motta O, De Caro F, Pagliano P. Sepsis—a retrospective cohort study of bloodstream infections. Antibiotics. 2020;9(12):851.
Zlatian O, Balasoiu AT, Balasoiu M, Cristea O, Docea AO, Mitrut R, et al. Antimicrobial resistance in bacterial pathogens among hospitalized patients with severe invasive infections. Exp Ther Med. 2018;16:4499–510.
Zhen X, Lundborg CS, Sun X, Hu X, Dong H. Economic burden of antibiotic resistance in ESKAPE organisms: a systematic review. Antimicrob Resist Infect Control. 2019;8:137.
Akin A, Alp E, Altindiş MU, Azak E, Batirel A, Çağ Y et al. Current diagnosis and treatment approach to sepsis. Mediterr J Infect Microbes Antimicrob. 2018;7:1–19.
Feng M, Sun T, Zhao Y, Zhang H. Detection of serum interleukin-6/10/18 levels in sepsis and its clinical significance. J Clin Lab Anal. 2016;6:1037–43.
Gyawali B, Ramakrishna K, Dhamoon AS. Sepsis: the evolution in definition, pathophysiology, and management. SAGE Open Med. 2019;2050312119835043. https://doi.org/10.1177/2050312119835043.
Mira JC, Cuschieri J, Ozrazgat-Baslanti T, Wang Z, Ghita GL, Loftus TJ, et al. The epidemiology of chronic critical illness after severe traumatic injury at two level one trauma centers. Crit Care Med. 2017;45:1989–96.
McMullan BJ, Mostaghim M. Prescribing azithromycin Aust Prescr. 2015;38:87–9.
FDA. Rocephin (Ceftriaxone injection). Genentech, USA. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/050585s068lbl.pdf. Accessed 17 Nov 2021.
Atkin SD, Abid S, Foster M, Bose M, Keller A, Hollaway R, et al. Multidrug-resistant Pseudomonas aeruginosa from sputum of patients with cystic fibrosis demonstrates a high rate of susceptibility to ceftazidime–avibactam. Infect Drug Resist. 2018;11:1499–510.
Bonomo RA, Donskey CJ, Blumer JL, Hujer AM, Hoyenm CK, Jacobs MR, et al. Cefotaxime-resistant bacteria colonizing older people admitted to an acute care hospital. J Am Geriat Soc. 2003;51:519–22.
Afriyie DK, Adu LB, Dzradosi M, Amponsah SK, Ohene-Manu P, Manu-Ofei F. Comparative in vitro activity of ciprofloxacin and levofloxacin against isolated uropathogens in Ghana: a pilot study. Pan Afr Med J. 2018;30. https://doi.org/10.11604/pamj.2018.30.194.15457
Stein M, Komerska J, Prizade M, Sheinberg B, Tasher D, Somekh E. Clindamycin resistance among Staphylococcus aureus strains in Israel: implications for empirical treatment of skin and soft tissue infections. Int J Infect Dis. 2016;46:18–21.
CDC. Carbapenem resistant Enterobacterals. 2019. https://www.cdc.gov/hai/organisms/cre/index.html. Accessed 17 Nov 2021.
Jang WH, Yoo DH, Park SW. Prevalence of and risk factors for levofloxacin-resistant E. coli isolated from outpatients with urinary tract infection. Korean J Urol. 2011;52:554–9.
Levitus M, Rewane A, Perera TB. Vancomycin-resistant enterococci. [Updated 2022 May 15]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513233/
Banin E, Hughes D, Kuipers OP. Bacterial pathogens, antibiotics and antibiotic resistance. FEMS microbial Rev. 2017;41:450–2.
Esposito S, De Simone G, Boccia G, De Caro F, Pagliano P. Sepsis and septic shock: new definitions, new diagnostic and therapeutic approaches. J Glob Antimicrob Resist. 2017;10:204–12.
Minasyan H. Sepsis: mechanisms of bacterial injury to the patient. Scand J Trauma Resusc Emerg Med. 2019;27:1–22.
Subramaniam S, Joyce P, Thomas N, Prestidge CA. Bioinspired drug delivery strategies for repurposing conventional antibiotics against intracellular infections. Adv Drug Deliv Rev. 2021;177:113948.
Yin W, Wang Y, Liu L, He J. Biofilms: the microbial “protective clothing” in extreme environments. Int J Mol Sci. 2019;20:3423.
Vestby LK, Grønseth T, Simm R, Nesse LL. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics. 2020;9:59.
Kvich L, Burmølle M, Bjarnsholt T, Lichtenberg M. Do mixed-species biofilms dominate in chronic infections?–need for in situ visualization of bacterial organization. Front Cell Infect Microbiol. 2020;10:396.
Thakur A, Mikkelsen H, Jungersen G. Intracellular pathogens: host immunity and microbial persistence strategies. J Immunol Res. 2019;2019:1356540. https://doi.org/10.1155/2019/1356540
Wright KJ, Seed PC, Hultgren SJ. Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cell Microbiol. 2007;9:2230–41.
Edwards AM, Potts JR, Josefsson E, Massey RC. Staphylococcus aureus host cell invasion and virulence in sepsis is facilitated by the multiple repeats within FnBPA. PLoS pathog. 2010;6:e1000964.
Horsley H, Malone-Lee J, Holland D, Tuz M, Hibbert A, Kelsey M, Kupelian A, Rohn JL. Enterococcus faecalis subverts and invades the host urothelium in patients with chronic urinary tract infection. PLoS ONE. 2013;8:e83637.
Bergmann R, Gulotta G, Andreoni F, Sumitomo T, Kawabata S, Zinkernagel AS, Chhatwal GS, Nizet V, Rohde M, Uchiyama S. The group A Streptococcus interleukin-8 protease SpyCEP promotes bacterial intracellular survival by evasion of autophagy. Infect Microbe Dis. 2022;4(3):116–23.
Peterson E, Kaur P. Antibiotic resistance mechanisms in bacteria: relationships between resistance determinants of antibiotic producers, environmental bacteria, and clinical pathogens. Front Microbiol. 2018;9:2928.
Abdul-Jabar HH, Abd AH, Abdulamir AS. Efficacy of combinations of piperacilline/tazobactam, ceftazidime, amikacin and bacteriophage against Enterobacteriaceae sepsis in neonates: in vitro study. SRP. 2020;11:165–70.
Brunkhorst FM, Oppert M, Marx G, Bloos F, Ludewig K, Putensen C, et al. Effect of empirical treatment with moxifloxacin and meropenem vs meropenem on sepsis-related organ dysfunction in patients with severe sepsis: a randomized trial. JAMA. 2012;307:2390–9.
Martínez ML, Plata-Menchaca EP, Ruiz-Rodríguez JC, Ferrer R. An approach to antibiotic treatment in patients with sepsis. J Thorac Dis. 2020;12:1007–21.
Thomson KM, Dyer C, Liu F, Sands K, Portal E, Carvalho MJ, et al. Effects of antibiotic resistance, drug target attainment, bacterial pathogenicity and virulence, and antibiotic access and affordability on outcomes in neonatal sepsis: an international microbiology and drug evaluation prospective substudy. Lancet Infect Dis. 2021;21:1677–88.
Waltner-Toews RI, Paterson DL, Qureshi ZA, Sidjabat HE, Adams-Haduch JM, Shutt KA, et al. Clinical characteristics of bloodstream infections due to ampicillin-sulbactam-resistant, non-extended-spectrum-β-lactamase-producing Escherichia coli and the role of TEM-1 hyperproduction. Antimicrob Agents Chemother. 2011;55:495–501.
Martínez-Casanova J, Gómez-Zorrilla S, Prim N, Dal Molin A, Echeverría-Esnal D, Gracia-Arnillas MP, et al. Risk factors for amoxicillin-clavulanate resistance in community-onset urinary tract infections caused by Escherichia coli or Klebsiella pneumoniae: the role of prior exposure to fluoroquinolones. Antibiotics. 2021;10:582.
Liu X, Zheng H, Zhang W, Shen Z, Zhao M, Chen Y, Sun L, Shi J, Zhang J. Tracking cefoperazone/sulbactam resistance development in vivo in A. baumannii isolated from a patient with hospital-acquired pneumonia by whole-genome sequencing. Front Microbiol. 2016;7:1268.
Hubbard A, Mason J, Roberts P, Parry CM, Corless C, van Aartsen J, Howard A, Bulgasim I, Fraser AJ, Adams ER, Roberts AP. Piperacillin/tazobactam resistance in a clinical isolate of Escherichia coli due to IS26-mediated amplification of blaTEM-1B. Nat Commun. 2020;11:1–9.
Gangcuangco LM, Alejandria M, Henson KE, Alfaraz L, Ata RM, Lopez M, et al. Prevalence and risk factors for trimethoprim–sulfamethoxazole-resistant Escherichia coli among women with acute uncomplicated urinary tract infection in a developing country. Int J Infect Dis. 2015;34:55–60.
Farha MA, Brown ED. Drug repurposing for antimicrobial discovery. Nat Microbiol. 2019;4:565–77.
Miró-Canturri A, Ayerbe-Algaba R, Smani Y. Drug repurposing for the treatment of bacterial and fungal infections. Front Microbiol. 2019;10:41. https://doi.org/10.3389/fmicb.2019.00041
Quezada H, Martínez-Vázquez M, López-Jácome E, González-Pedrajo B, Andrade Á, Fernández-Presas AM, et al. Repurposed anti-cancer drugs: the future for anti-infective therapy? Expert Rev Anti Infect Therapy. 2020;18:609–12.
Hegazy WA, Khayat MT, Ibrahim TS, Nassar MS, Bakhrebah MA, Abdulaal WH, Alhakamy NA, Bendary MM. Repurposing anti-diabetic drugs to cripple quorum sensing in Pseudomonas aeruginosa. Microorganisms. 2020;8(9):1285.
US National Library of Medicine. The efficacy and safety of Ta1 for sepsis (TESTS). 2021. https://clinicaltrials.gov/ct2/show/NCT02867267. Accessed 17 Nov 2021.
Francois B, Jeannet R, Daix T, Walton AH, Shotwell MS, Unsinger J, Monneret G, Rimmelé T, Blood T, Morre M, Gregoire A. Interleukin-7 restores lymphocytes in septic shock: the IRIS-7 randomized clinical trial. JCI Insight. 2018;3(5):e98960. https://doi.org/10.1172/jci.insight.98960.
Choy EH, De Benedetti F, Takeuchi T, Hashizume M, John MR, Kishimoto T. Translating IL-6 biology into effective treatments. Nat Rev Rheumatol. 2020;16:335–45.
Honore PM, Hoste E, Molnár Z, Jacobs R, Joannes-Boyau O, Malbrain ML, et al. Cytokine removal in human septic shock: where are we and where are we going? Ann Intensive Care. 2019;9:56.
Yuan H, Ma Q, Ye L, Piao G. The traditional medicine and modern medicine from natural products. Molecules. 2016;21(5):559. https://doi.org/10.3390/molecules21050559.
Alikiaii B, Bagherniya M, Askari G, Johnston TP, Sahebkar A. The role of phytochemicals in sepsis: a mechanistic and therapeutic perspective. BioFactors. 2021;47:19–40.
Jhanji R, Singh A, Kumar A. Antibacterial potential of selected phytomolecules: an experimental study. Microbiol Immunol. 2021;65:325–32.
Wojtyczka RD, Dziedzic A, Kępa M, Kubina R, Kabała-Dzik A, Mularz T, et al. Berberine enhances the antibacterial activity of selected antibiotics against coagulase-negative Staphylococcus strains in vitro. Molecules. 2014;19:6583–96.
Xia X, Yan J, Shen Y, Tang K, Yin J, Zhang Y, Yang D, Liang H, Ye J, Weng J. Berberine improves glucose metabolism in diabetic rats by inhibition of hepatic gluconeogenesis. PLoS one. 2011;6(2):e16556. https://doi.org/10.1371/journal.pone.0016556
Siriyong T, Srimanote P, Chusri S, Yingyongnarongkul BE, Suaisom C, Tipmanee V, et al. Conessine as a novel inhibitor of multidrug efflux pump systems in Pseudomonas aeruginosa. BMC Complement Altern Med. 2017;17:1–7.
Mgbeahuruike EE, Stålnacke M, Vuorela H, Holm Y. Antimicrobial and synergistic effects of commercial piperine and piperlongumine in combination with conventional antimicrobials. Antibiotics. 2019;8:55.
Ndezo B, Tokam Kuaté CR, Dzoyem JP. Synergistic antibiofilm efficacy of thymol and piperine in combination with three aminoglycoside antibiotics against Klebsiella pneumoniae biofilms. Can J Infect Dis Med Microbiol. 2021;2021:1–8. https://doi.org/10.1155/2021/7029944.
Hamoud R, Reichling J, Wink M. Synergistic antibacterial activity of the combination of the alkaloid sanguinarine with EDTA and the antibiotic streptomycin against multidrug resistant bacteria. J Pharm Pharmacol. 2015;67:264–73.
Lu C, Zhang N, Kou S, Gao L, Peng B, Dai Y, et al. Sanguinarine synergistically potentiates aminoglycoside-mediated bacterial killing. Microb Biotechnol. 2022;15:2055–70.
Mun SH, Lee YS, Han SH, Lee SW, Cha SW, Kim SB, et al. In vitro potential effect of morin in the combination with β-lactam antibiotics against methicillin-resistant Staphylococcus aureus. Foodborne Pathog Dis. 2015;12:545–50.
Amin MU, Khurram M, Khattak B, Khan J. Antibiotic additive and synergistic action of rutin, morin and quercetin against methicillin resistant Staphylococcus aureus. BMC Complement Altern Med. 2015;15:1–2.
Deepika MS, Thangam R, Sakthidhasan P, Arun S, Sivasubramanian S, Thirumurugan R. Combined effect of a natural flavonoid rutin from Citrus sinensis and conventional antibiotic gentamicin on Pseudomonas aeruginosa biofilm formation. Food Control. 2018;90:282–94.
Alnour TM, Ahmed-Abakur EH, Elssaig EH, Abuduhier FM, Ullah MF. Antimicrobial synergistic effects of dietary flavonoids rutin and quercetin in combination with antibiotics gentamicin and ceftriaxone against E. coli (MDR) and P. mirabilis (XDR) strains isolated from human infections: implications for food–medicine interactions. Ital J Food Sci. 2022;34:34–42.
Siriwong S, Teethaisong Y, Thumanu K, Dunkhunthod B, Eumkeb G. The synergy and mode of action of quercetin plus amoxicillin against amoxicillin-resistant Staphylococcus epidermidis. BMC Pharmacol Toxicol. 2016;17:1–4.
Pal A, Tripathi A. Demonstration of bactericidal and synergistic activity of quercetin with meropenem among pathogenic carbapenem resistant Escherichia coli and Klebsiella pneumoniae. Microb Pathog. 2020;143:104120.
Cai W, Fu Y, Zhang W, Chen X, Zhao J, Song W, et al. Synergistic effects of baicalein with cefotaxime against Klebsiella pneumoniae through inhibiting CTX-M-1 gene expression. BMC Microbiol. 2016;16:1–9.
Siriwong S, Pimchan T, Naknarong W, Eumkeb G. Mode of action and synergy of ceftazidime and baicalein against Streptococcus pyogenes. Trop J Pharm Res. 2015;14:641–8.
Sivananthan M, Imanina CW. Synergistic activity of chloroform and methanol extract of Andrographis paniculata with erythromycin against Streptococcus agalactiae. Int J Pharmtech Res. 2013;5:1393–8.
Zhang G, Jiang C, Xie N, Xu Y, Liu L, Liu N. Treatment with andrographolide sulfonate provides additional benefits to imipenem in a mouse model of Klebsiella pneumoniae pneumonia. Biomed Pharmacother. 2019;117:109065.
Veras HN, Rodrigues FF, Botelho MA, Menezes IR, Coutinho HD, Costa JG. Enhancement of aminoglycosides and β-lactams antibiotic activity by essential oil of Lippia sidoides Cham. and the thymol. Arab J Chem. 2017;10:2790–5.
Miladi H, Zmantar T, Kouidhi B, Al Qurashi YM, Bakhrouf A, Chaabouni Y, et al. Synergistic effect of eugenol, carvacrol, thymol, p-cymene and γ-terpinene on inhibition of drug resistance and biofilm formation of oral bacteria. Microb Pathog. 2017;112:156–63.
Vázquez NM, Fiorilli G, Guido PA, Moreno S. Carnosic acid acts synergistically with gentamicin in killing methicillin-resistant Staphylococcus aureus clinical isolates. Phytomedicine. 2016;23:1337–43.
Mun SH, Kang OH, Kong R, Zhou T, Kim SA, Shin DW, et al. Punicalagin suppresses methicillin resistance of Staphylococcus aureus to oxacillin. J Pharmacol Sci. 2018;137:317–23.
Dey D, Ghosh S, Ray R, Hazra B. Polyphenolic secondary metabolites synergize the activity of commercial antibiotics against clinical isolates of β-lactamase-producing Klebsiella pneumoniae. Phytother Res. 2016;30:272–82.
Chaves SKM, Feitosa CM, da Araújo LS. Alkaloids pharmacological activities-prospects for the development of phytopharmaceuticals for neurodegenerative diseases. Curr Pharm Biotechnol. 2016;17:629–35.
Peng L, Kang S, Yin Z, Jia R, Song X, Li L, et al. Antibacterial activity and mechanism of berberine against Streptococcus agalactiae. Int J Clin Exp Pathl. 2015;8:5217–23.
Daglia M. Polyphenols as antimicrobial agents. Curr Opin Biotechnol. 2012;23:174–81.
Matilla-Cuenca L, Gil C, Cuesta S, Rapún-Araiz B, Žiemytė M, Mira A, et al. Antibiofilm activity of flavonoids on staphylococcal biofilms through targeting BAP amyloids. Sci Rep. 2020;10:18968.
Sun LC, Li SY, Wang FZ, Xin FJ. Research progresses in the synthetic biology of terpenoids. Biotechnol Bull. 2017;33:64.
Lopez-Romero JC, González-Ríos H, Borges A, Simões M. Antibacterial effects and mode of action of selected essential oils components against Escherichia coli and Staphylococcus aureus. Evid Based Complement Alternat Med. 2015;2015:795435. https://doi.org/10.1155/2015/795435.
Farha AK, Yang QQ, Kim G, Li HB, Zhu F, Liu HY, et al. Tannins as an alternative to antibiotics. Food Biosci. 2020;38:100751.
Serrano J, Puupponen-Pimiä R, Dauer A, Aura AM, Saura-Calixto F. Tannins: current knowledge of Ndefood sources, intake, bioavailability and biological effects. Mol Nutr Food Res. 2009;53:310–29.
Cai X, YU X. Synergetic effect of Xuebijing injection and cefoperazone sodium/sulbactam sodium on sepsis. China Pharmacy. 2005;20.
Eslami K, Mahmoodpoor A, Ahmadi A, Abdollahi M, Kamali K, Mousavi S, Najafi A, Baeeri M, Hamishehkar H, Kouti L, Javadi MR. Positive effect of septimeb™ on mortality rate in severe sepsis: a novel non antibiotic strategy. DARU J Pharmaceut Sci. 2012;20:1–7.
Gupta PD, Birdi TJ. Development of botanicals to combat antibiotic resistance. J Ayurveda Integr Med. 2017;8:266–75.
Kongkham B, Prabakaran D, Puttaswamy H. Opportunities and challenges in managing antibiotic resistance in bacteria using plant secondary metabolites. Fitoterapia. 2020;147:104762. https://doi.org/10.1016/j.fitote.2020.104762.
Rubio I, Osuchowski MF, Shankar-Hari M, Skirecki T, Winkler MS, Lachmann G, et al. Current gaps in sepsis immunology: new opportunities for translational research. Lancet Infect Dis. 2019;19:422–36.
Chinowaita F, Chaka W, Nyazika TK, Maboreke TC, Tizauone E, Mapondera P, et al. Sepsis in cancer patients residing in Zimbabwe: spectrum of bacterial and fungal aetiologies and their antimicrobial susceptibility patterns. BMC Infect Dis. 2020;20:161.
Ferreira JN, Correia LR, Oliveira RM, Watanabe SN, Possari JF, Lima AF. Managing febrile neutropenia in adult cancer patients: an integrative review of the literature. Rev Bras Enferm. 2017;70:1301–8.
Lemiale V, Pons S, Mirouse A, Tudesq JJ, Hourmant Y, Mokart D, et al. Sepsis and septic shock in patients with malignancies: a Groupe de Recherche Respiratoire en Réanimation Onco-Hématologique study. Crit Care Med. 2020;48:822–9.
Liu Z, Mahale P, Engels EA. Sepsis and risk of cancer among elderly adults in the United States. Clin Infect Dis. 2019;68:717–24.
Gudiol C, Albasanz-Puig A, Cuervo G, Carratalà J. Understanding and managing sepsis in patients with cancer in the era of antimicrobial resistance. Front Med. 2021;8:636547. https://doi.org/10.3389/fmed.2021.636547.
Subburaj D, Uppuluri R, Jayaraman D, Vellaichamyswaminathan V, Kandath S, Raj R. Combating blood stream infections during induction chemotherapy in children with acute myeloid leukemia: single center results in India. Pediatr Blood Cancer. 2017;64(10):e26517.
Jacob S, Jacob SE, Suryanarayana BS, Dutta TK. Clinical profile and short term outcome of adult patients with acute myeloid leukemia. Indian J Hematol Blood Transfus. 2019;35:431–6.
Papanicolas LE, Gordon DL, Wesselingh SL, Rogers GB. Not just antibiotics: is cancer chemotherapy driving antimicrobial resistance? Trends Microbiol. 2018;26:393–400.
Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: a review of pathogenesis. Indian J Endocrinol Metab. 2012;16:27–36.
Frydrych LM, Fattahi F, He K, Ward PA, Delano MJ. Diabetes and sepsis: risk, recurrence, and ruination. Front Endocrinol. 2017;8:271. https://doi.org/10.3389/fendo.2017.00271.
Mor A, Dekkers OM, Nielsen JS, Beck-Nielsen H, Sørensen HT, Thomsen RW. Impact of glycemic control on risk of infections in patients with type 2 diabetes: a population-based cohort study. Am J Epidemiol. 2017;186:227–36.
Steinhagen F, Schmidt SV, Schewe JC, Peukert K, Klinman DM, Bode C. Immunotherapy in sepsis-brake or accelerate?. Pharmacol Ther. 2020;208:107476.
Liew KY, Hafiz MF, Chong YJ, Harith HH, Israf DA, Tham CL. A review of malaysian herbal plants and their active constituents with potential therapeutic applications in sepsis. Evid Based Complement Alternat Med. 2020;2020:8257817. https://doi.org/10.1155/2020/8257817.
Li F, Li XM, Sheng D, Chen SR, Nie X, Liu Z, et al. Discovery and preliminary SAR of 14-aryloxy-andrographolide derivatives as antibacterial agents with immunosuppressant activity. RSC Adv. 2018;8:9440–56.
Yang W, Chen X, Li Y, Guo S, Wang Z, Yu X. Advances in pharmacological activities of terpenoids. Nat Prod Commun. 2020;15. https://doi.org/10.1177/1934578X20903555
Yu Y, Shen Q, Lai Y, Park SY, Ou X, Lin D, et al. Anti-inflammatory effects of curcumin in microglial cells. Front Pharmacol. 2018;9:386.
Chai YS, Chen YQ, Lin SH, Xie K, Wang CJ, Yang YZ, et al. Curcumin regulates the differentiation of naïve CD4+ T cells and activates IL-10 immune modulation against acute lung injury in mice. Biomed Pharmacother. 2020;125:109946.
Wang X, Feng S, Ding N, He Y, Li C, Li M, et al. Anti-inflammatory effects of berberine hydrochloride in an LPS-induced murine model of mastitis. Evid Based Complement Alternat Med. 2018;2018:5164314. https://doi.org/10.1155/2018/5164314.
Li C, Xi Y, Li S, Zhao Q, Cheng W, Wang Z, et al. Berberine ameliorates TNBS induced colitis by inhibiting inflammatory responses and Th1/Th17 differentiation. Mol Immunol. 2015;67:444–54.
Okubo S, Uto T, Goto A, Tanaka H, Nishioku T, Yamada K, et al. Berberine induces apoptotic cell death via activation of caspase-3 and-8 in HL-60 human leukemia cells: nuclear localization and structure–activity relationships. Am J Chin Med. 2017;45:1497–511.
D’Alesio C, Bellese G, Gagliani MC, Aiello C, Grasselli E, Marcocci G, et al. Cooperative antitumor activities of carnosic acid and trastuzumab in ERBB2+ breast cancer cells. J Exp Clin Cancer Res. 2017;36:1–6.
Talib WH. Regressions of breast carcinoma syngraft following treatment with piperine in combination with thymoquinone. Sci Pharm. 2017;85:27.
Zhang L, Chinnathambi A, Alharbi SA, Veeraraghavan VP, Mohan SK, Zhang G. Punicalagin promotes the apoptosis in human cervical cancer (ME-180) cells through mitochondrial pathway and by inhibiting the NF-kB signaling pathway. Saudi J Biol Sci. 2020;27:1100–6.
Olajide OA, Kumar A, Velagapudi R, Okorji UP, Fiebich BL. Punicalagin inhibits neuroinflammation in LPS-activated rat primary microglia. Mol Nutr Food Res. 2014;58:1843–51.
Koren-Gluzer M, Aviram M, Meilin E, Hayek T. The antioxidant HDL-associated paraoxonase-1 (PON1) attenuates diabetes development and stimulates β-cell insulin release. Atherosclerosis. 2011;219:510–8.
Chekalina N, Burmak Y, Petrov Y, Borisova Z, Manusha Y, Kazakov Y, et al. Quercetin reduces the transcriptional activity of NF-kb in stable coronary artery disease. Indian Heart J. 2018;70:593–7.
Carrasco-Pozo C, Tan KN, Reyes-Farias M, De La Jara N, Ngo ST, Garcia-Diaz DF, Llanos P, Cires MJ, Borges K. The deleterious effect of cholesterol and protection by quercetin on mitochondrial bioenergetics of pancreatic β-cells, glycemic control and inflammation: in vitro and in vivo studies. Redox Biol. 2016;9:229–43.
Mussard E, Cesaro A, Lespessailles E, Legrain B, Berteina-Raboin S, Toumi H. Andrographolide, a natural antioxidant: an update. Antioxidants. 2019;8(12):571. https://doi.org/10.3390/antiox8120571.
Zhang L, Bao M, Liu B, Zhao H, Zhang Y, Ji X, et al. Effect of andrographolide and its analogs on bacterial infection: a review. Pharmacology. 2020;105:123–34.
Habtemariam S. Berberine pharmacology and the gut microbiota: a hidden therapeutic link. Pharmacol Res. 2020;155:104722.
Zheng D, Huang C, Huang H, Zhao Y, Khan MR, Zhao H, et al. Antibacterial mechanism of curcumin: a review. Chem Biodivers. 2020;17:e2000171.
Karimi A, Ghodsi R, Kooshki F, Karimi M, Asghariazar V, Tarighat-Esfanjani A. Therapeutic effects of curcumin on sepsis and mechanisms of action: a systematic review of preclinical studies. Phytother Res. 2019;33:2798–820.
Sundaramoorthy NS, Sivasubramanian A, Nagarajan S. Simultaneous inhibition of MarR by salicylate and efflux pumps by curcumin sensitizes colistin resistant clinical isolates of Enterobacteriaceae. Microb Pathog. 2020;148:104445.
Tyagi P, Singh M, Kumari H, Kumari A, Mukhopadhyay K. Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PloS one. 2015;10. https://doi.org/10.1371/journal.pone.0121313
Gosset-Erard C, Zhao M, Lordel-Madeleine S, Ennahar S. Identification of punicalagin as the bioactive compound behind the antimicrobial activity of pomegranate (Punica granatum L.) peels. Food Chem. 2021;352:129396.
Cao Y, Chen J, Ren G, Zhang Y, Tan X, Yang L. Punicalagin prevents inflammation in LPS-induced RAW264. 7 macrophages by inhibiting FoxO3a/autophagy signaling pathway. Nutrients. 2019;11:2794.
Xu Y, Shi C, Wu Q, Zheng Z, Liu P, Li G, et al. Antimicrobial activity of punicalagin against Staphylococcus aureus and its effect on biofilm formation. Foodborne Pathog Dis. 2017;14:282–7.
David AV, Arulmoli R, Parasuraman S. Overviews of biological importance of quercetin: a bioactive flavonoid. Pharmacogn Rev. 2016;10:84–9.
Chen T, Zhang X, Zhu G, Liu H, Chen J, Wang Y, He X. Quercetin inhibits TNF-α induced HUVECs apoptosis and inflammation via downregulating NF-kB and AP-1 signaling pathway in vitro. Medicine. 2020;99(38):e22241.
Vipin C, Mujeeburahiman M, Ashwini P, Arun AB, Rekha PD. Anti-biofilm and cytoprotective activities of quercetin against Pseudomonas aeruginosa isolates. Lett Appl Microbiol. 2019;68:464–71.
Author information
Authors and Affiliations
Contributions
Acquisition and analysis of data, writing the original draft, and the artwork were done by Parkhi Shrivastava. Reviewing the manuscript and preparing the final draft were done by Ragini Gothalwal. Conceptualization of idea and creation of model, analysis/interpretation of data, writing and editing the manuscript, and supervision of the work were done by Puneet Gandhi. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shrivastava, P., Gothalwal, R. & Gandhi, P. Therapeutic Strategies to Ameliorate Antibiotic Resistance and Host-Inflammation Response in Sepsis: an Innovative Approach. Curr Clin Micro Rpt 10, 85–98 (2023). https://doi.org/10.1007/s40588-023-00194-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40588-023-00194-6