Abstract
Purpose of Review
During infection, the human fungal pathogen Cryptococcus neoformans undergoes an unusual change in size, from small haploid yeast to large polyploid Titan cells. This transition is now well recognized as a virulence factor, but significant questions remain about how Titanisation is regulated and how it influences disease progression. Progress has been impeded by the lack of an in vitro model for the yeast-to-Titan transition, a challenge that was recently overcome by three independent groups.
Recent Findings
Here, we review Titanization in the context of patient samples and animal models and set the stage for three new reports describing in vitro Titan cell induction assays. We compare and contrast key findings, place them in the broader research context, and identify areas of further interest.
Summary
New in vitro models will allow pressing questions about molecular mechanisms driving the yeast-to-Titan transition and their influence on drug resistance and pathogenesis to be addressed.
Similar content being viewed by others
Introduction
Fungal infections are an underappreciated threat to global health, causing an estimated 1.5 million deaths each year [1]. Infection with Cryptococcus species remains a leading cause of morbidity and mortality among both immunocompromised and immunocompetent individuals [1, 2]. Disease occurs when desiccated yeast or spores are inhaled, proliferate in the lung, and disseminate to the central nervous system (CNS), causing life-threatening meningitis [3, 4]. A key determinate of disease progression is the Cryptococcus Titan cell, a striking fungal morphotype induced in the lung by host-relevant factors [5, 6••, 7••, 8, 9] (Fig. 1). This review addresses common questions surrounding this topic and highlights recent developments in our understanding of the cryptococcal yeast-to-Titan transition that reveal underlying mechanisms of pathogenesis, drug resistance, and immune evasion.
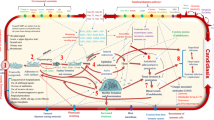
Cryptococcus neoformans grows as a heterogeneous population of yeast and Titan cells. a In vitro-induced yeast and Titan cells counterstained with India ink to reveal capsule. b In vitro-induced yeast (black arrows) and Titan (white arrows) cells co-cultured with J77.4 macrophage-like cells. c A schematic showing yeast- and Titan-phase cell cycles in the environment and in the host lung. d A budding Titan mother with asymmetric DNA division (blue = calcofluor white, chitin; red = Cse4-mCherry, chromosomes)
Morphological transitions by fungi are hallmarks of pathogenicity that enable growth in the host microenvironment, mediate tissue damage, and influence immune evasion and immune cell recruitment [10,11,12]. Following inhalation of spores into the lung, or upon in vitro induction, members of the Cryptococcus species complex (C. neoformans, C. deneoformans, C. gattii) undergo a dramatic change in cell size from small (5–7 μm) yeast to large (> 10–100 μm) Titan cells, evading phagocytosis [6••, 7••, 8, 9, 13] (Fig. 1a, b). During this transition, yeasts grow isotropically and undergo repeated rounds of endo-reduplication to form large, highly polyploid Titan cells with distinct morphology and cell cycle (Fig. 1c). These large cells divide their DNA asymmetrically and produce disproportionately small haploid, diploid, or aneuploid daughter cells [6••, 14•] (Fig. 1d). The result is an overall population that is heterogeneous for both ploidy and size, and this heterogeneity has important implications for drug resistance and immune evasion [14•].
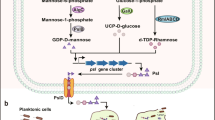
Despite their important roles in pathogenesis, Titan cells were unrecognized as a feature of cryptococcosis until 2010 and could not be reliably generated in vitro until 2018 [6••, 7••, 15, 16••, 17]. Our lab, together with two other groups, has recently demonstrated that the yeast-to-Titan transition can be reproducibly triggered by growth in minimal medium and exposure to an inducing condition: serum containing bacterial cell wall, serum supplemented with sodium azide, or continuous mixing under hypoxia (Fig. 2) [6••, 7••, 16••]. The identification of in vitro inducing conditions, coupled with previous in vivo and in vitro work identifying regulatory signaling pathways, demonstrates that the yeast-to-Titan transition is a regulated developmental switch analogous to the yeast-to-hyphal transition of dimorphic fungi [6••, 18,19,20,21]. However, significant questions remain about the basic cell biology of this morphotype, and how it influences host-pathogen interactions.
Schematic showing three new in vitro Titan cell induction protocols. a Induction of Titan cells through pre-growth in minimal medium followed by exposure to 10% serum. b Induction of Titan-like cells through pre-growth in minimal medium followed by exposure to serum + sodium azide. c Induction of Titan cells through continuous shaking under hypoxia. Relevant references for each method are indicated
How Frequently Are Titan Cells Observed in Patient Isolates?
Clinical reports of Titan cells can be found in the literature as early as 1973, when large encapsulated yeasts were isolated from the lung of a young African woman [22]. In 1985, Love et al. again reported unusually large, dimorphic cells in isolates directly from the brain of a young African man [23]. In each case, Titans were observed only immediately after isolation from the patient: large cells converted back to producing a typical homogenous yeast population upon in vitro culture on Sabouraud or brain heart infusion agar. When mice were infected with these strains, but not a laboratory control, atypical large cells were again observed [22, 23]. Morphologically, these large cells were reported to produce a distinct capsule and cell wall structure compared to their yeast-phase counterparts, consistent with formal descriptions of Titan cells [8, 9, 22]. However, these early isolates were dismissed as atypical until 2010 [8, 24].
Perhaps contributing to this underappreciated role, C. neoformans Titan cells have not been reported in patient cerebral spinal fluid (CSF), classically one of the most important sites for diagnosis of cryptococcosis. A 1985 report of serial CSF isolates from a patient with lupus notes that cryptococcal cells directly observed from the CSF were heterogeneous in cell size, and classified these cells as having either distinct or unstructured organelles following TEM [25]. While this is consistent with TEM of in vitro and in vivo Titan cells [6••, 9], the few available documented cells from these CSF isolates fail to reach the > 10-μm threshold [25]. A subsequent 2014 analysis of serial CSF samples from 134 patients likewise failed to identify cells > 10 μm [26]. However, these authors did observe a subset of patients with a significantly larger overall median cell body size of 8.2 μm and reported cells > 30 μm including capsule. This highlights a key challenge when identifying Titan cells in patient samples: Current definitions use arbitrary size cutoffs and differ on whether to include capsule diameter, rather than relying on a complete analysis of key morphological features [12, 15, 27]. It remains to be seen whether cryptococcal cells in the CSF truly do not form Titan cells or whether they undergo the yeast-to-Titan transition in a limited way but do not cross the > 10-μm threshold. One intriguing data point to consider is that cells exposed to continuous mixing (800 rpm/~ 1 g) in minimal medium and hypoxia form Titan-like cells at a high rate in vitro (Fig. 2c) [7••]. This may be analogous to continuous mixing by CSF flow that cells are likely to be exposed to in the CNS, a nutritionally limited, hypoxic environment [28,29,30].
How Do We Define a Titan Cell?
As mentioned above, in vivo-derived Titan cells exhibit clear morphological and ploidy alterations relative to yeast [8, 18]. In vivo, Titans can reach up to 100 μm, while typical yeast cells are around 5 μm [8]. However, several authors have established conflicting definitions of the minimum size threshold, relying on arbitrary size cutoffs and differing on whether to include capsule diameter [12, 15, 27]. In addition to cell size, Titans exhibit increased cell wall thickness and maintain a characteristic single, large, intracellular vacuole that occupies the majority of the intracellular space [9]. Increased cell size is associated with increased DNA content: in vivo Titan cells are highly polyploid—often tetraploid or octoploid, but reaching as high as 64C—and they can produce diploid or aneuploid daughters with normal cell size [8, 9, 14•]. In addition, Titan cells exhibit changes in important virulence factors: they have highly compacted polysaccharide capsule and altered pathogen-associated molecular pattern (PAMP) exposure, including altered chitin and chitosan content in the cell wall [9, 31, 32]. Titan cells are also are less frequently phagocytosed and are more resistant to lung phagocyte killing and nitrosative and oxidative stress [8].
In vitro induction protocols have established a more parsimonious definition for Titans with four key criteria: (1) cell body size > 10 μm; (2) cell ploidy > 2C; (3) the presence of a single, large vacuole; and (4) altered cell wall and capsule [6••, 7••]. However, these criteria may require further refinement. For example, despite inclusion of the capsule in the Titan cell definition, capsule itself is not required for Titanization [6••]. In addition, definitions based on increased mother ploidy (> 2C) and cell size (> 10 μm) should also include asymmetric division of DNA and the production of disproportionately small daughter cells. Large “Titan-like” cells (> 10 μm) divide DNA symmetrically and produce proportionate daughters, similar to yeast-phase budding [6••, 7••]. In some cases, these buds fail to fully divide, accumulating defects in cytokinesis [6••]. Finally, these definitions have relied on work with the C. neoformans H99 isolate, which may skew understanding of underlying diversity across the species complex [7••]. Future work should more fully define the yeast-to-Titan switch across clinical and environmental isolates. Despite these limitations, we will rely on these four criteria throughout this work.
How Do Titan Cells Influence Disease Outcome?
Typically, cryptococcosis patients are immunocompromised and present either with cryptococcal meningitis, diagnosed by visualization of yeast in CSF, or are Cryptococcus antigen (CrAg) positive upon diagnosis of HIV or during routine monitoring upon immune suppression [2, 33]. Cryptococcal meningitis arising from infection with C. neoformans accounts for 15% of HIV-related deaths [17, 34]. This represents an unacceptably high mortality rate: among HIV patients with low CD4+ counts, only approximately 6% are positive for cryptococcal antigen. Overall, an estimated 278,000 cases of cryptococcal meningitis occur annually in HIV-positive patients, with 65% mortality [34]. While HIV status is the most frequent predisposing factor, cryptococcosis is also associated with solid organ transplant, particularly in patients receiving T cell depletion therapy or calcineurin inhibitors [33]. Among solid organ transplant patients, the overall incidence of cryptococcosis is 5%, and mortality attributed to cryptococcosis in transplant patients is 14% [33, 35]. Importantly, up to 20% of C. neoformans-infected patients have no identifiable underlying disease and this trend is consistent worldwide [36,37,38].
Cryptococcus infection begins in the lung, but cryptococcal pneumonia is often neglected in estimates of morbidity and mortality. In non-HIV/non-transplant (NHNT) patients, as well as in cyclosporin-treated transplant patients, there is an increased incidence of cryptococcal pneumonia in the absence of disseminated infection [27, 35, 37, 39]. While CNS involvement is predictive of poor outcome, pulmonary infection can also be fatal: Cryptococcal pneumonia was diagnosed upon autopsy in 7% of a cohort of patients who succumbed to respiratory disease and can be the cause of rapid respiratory failure upon introduction of antifungal therapy in patients with HIV [40, 41]. Finally, infection with the emerging pathogen Cryptococcus gattii is characterized by pulmonary cryptococcoma [42]. Taken together, there is growing evidence that cryptococcal pneumonia is an under-recognized challenge that is both a precursor to cryptococcal meningitis and a predictor of morbidity and mortality in its own right [43].
How might Titan cells contribute to these different disease processes? Dissemination from the lung occurs either via transcytosis or paracellular crossing of the lung epithelium or via phagocytosis and Trojan Horse-type dissemination (recently reviewed by [4]). In vivo and in vitro observations suggest that the morphological transition from yeast to Titan cell may contribute to pulmonary vs. disseminated disease [5, 6••]. In murine models of infection, Titan cells are associated with escape from the lung, crossing of the blood-brain barrier, and a non-protective immune response [5, 27, 32, 44]. Mice infected with low-Titanizing clinical isolates or mutants with Titanization defects exhibit differences in disease progression [5, 6••]. In these mice, dissemination to the brain is delayed, if not entirely prevented. However, it is not simply that Titans enable dissemination: Infection with cells that are hyper-Titanizing (otc1Δ or usv101Δ) prolongs the pulmonary phase and delays dissemination [5, 6••, 45].
How can these two observations be reconciled? First, Titans themselves are phagocytosed at low rates due to their large size (Fig. 1b) [8]. Thus, strains that produce more Titan cells are predicted to be less phagocytosed and disseminate more slowly (otc1Δ, usv101Δ). Moreover, daughters of Titan cells can themselves Titanize, further renewing the Titan population [6••]. In the case of the transcription factor USV101, a negative regulator of the yeast-to-Titan switch, we speculate that the progressive increase in the proportion of Titan cells relative to small cells generated by the usv101Δ mutant contributes to reduced dissemination to the brain [6••]. In addition, the presence of Titans reduces uptake of small cells through an unknown mechanism [9, 44]. However, under in vivo or in vitro Titan-inducing conditions, a heterogeneous population of daughter cells ranging from 2 to 7 μm is also observed [6••, 7••, 8, 9, 16••, 19]. These small cells simultaneously arise from normal yeast-phase budding and through asymmetric Titan budding and represent the majority of the infecting population [6••]. Additionally, Titan cells produce daughters at a more rapid rate (~ 60 min/bud) than yeast-phase cells [7••, 9]. Together, this increases the proportion of small daughter cells available to be phagocytosed and disseminated relative to a Titan-deficient strain. This may partially explain the association of Titans with dissemination, as both hypo-Titanizing and hyper-Titanizing mutants that exhibit reduced dissemination also show reduce lung CFUs compared to their wild-type parents [5, 45]. Perhaps the most significant finding is that, similar to Titans themselves, Titan daughters are more resistant to stress than their yeast-phase sisters, likely due to their altered ploidy [14•]. It remains to be seen whether Titan daughters undergo transcytosis or paracellular crossing at altered rates relative to yeast daughters, but it is clear that they are more resistant to killing by phagocytes and more likely to be drug resistant, thereby mediating pathogenesis.
Given the above differences in disease presentation in different populations, it is tempting to speculate that Titanizing strains are more likely to be observed in clinical compared to environmental isolates. However, when we examined the Titanization capacity of environmental isolates and clinical isolates from an HIV patient cohort in Zambia, we found no association with clinical or environmental origin or clade [6••, 46]. Likewise, the capacity to form Titans has been observed in all three major species (C. neoformans, C. deneoformans, C. gattii) [7••]. This points to the yeast-to-Titan transition being a conserved and widely occurring mechanism to facilitate adaptation to and evasion of environmental stress [6••].
How Are Titan Cells Induced?
One of the biggest challenges impeding efforts to understand the contribution of Titan cells to disease has been the lack of an in vitro model for their induction. Thanks to the work of three major collaborations spanning the field of Cryptococcus research, we now have three robust induction protocols that identify three independent inducing signals: microbial, host-derived, and physical (Fig. 2) [6••, 7••, 16••]. Continuing the tradition established by the original Titan cell papers, these three methods were published back to back to back in a single journal. Although there are differences in inducing signal between the various methods, there are also common themes, including cell density, nutrient availability, and oxygen limitation.
First, cell density has a clear and reproducible impact on Titanization across in vivo and in vitro models. Mice infected with a low multiplicity of infection (MOI) (104/ml) or presenting with asymptomatic infection exhibit a higher rate of enlarged cells [8, 9]. This was recapitulated in all three reports of in vitro Titan and Titan-like cell induction, where increased cell density reduces Titanization frequency and cell size [6••, 7••, 16••]. Interestingly, there appears to be a lower limit to the MOI effect, at least in the physical induction protocol (Fig. 2c): the frequency of cells > 10 μm is reduced at lower MOI (37% at 106 vs. 10% at 104) when induced by continuous mixing under hypoxia [16••]. However, induction frequency and size increase progressively at MOI < 106 in the presence of serum alone or serum + sodium azide in minimal medium at 37 °C in 5% CO2 (Fig. 2a, b) [6••, 16••].
One possible explanation for this is that density-dependent expression of secreted factors inhibits the yeast-to-Titan switch. Cryptococcus neoformans Qsp1 is a density-dependent secreted protein involved in protease expression and cell wall remodeling [47]. Loss of QSP1 results in an increase in the size and frequency of Titan cells during hypoxic growth with continuous mixing [16••]. We likewise observed an increase in the frequency of Titan cells produced by the qsp1Δ mutant in our assay (unpublished results). However, while Qsp1 activity partially explains suppression of Titanization at high density, deletion of qsp1Δ does not cause constitutive Titanization in the absence of an inducing signal [16••].
Another density-dependent signal, pantothenic acid (PA), may positively regulate Titanization, with the frequency of Titan cells increasing upon exposure to physiologically relevant sub-micromolar concentrations [16••]. However, exposure to micromolar concentrations inhibits Titanization. PA was originally identified as a factor in conditioned medium from stationary cultures that was able to increase growth rate in a density-dependent manner [48]. It remains to be seen whether the impact of PA on growth rate is sufficient to explain its impact on Titan frequency, or how PA interacts with Qsp1.
Second, low density alone is not sufficient to prime cells for Titanization: nutrient availability both before and during induction influences outcome (Fig. 2). When cells grown in rich medium (YPD) were used as the inoculating culture at low density (5 × 106 or 1 × 104/ml), true Titan cells were not observed, even when cells were washed thoroughly in PBS to remove any secreted signal [6••]. Rather, large cells (> 10 μm) that produced proportionally sized daughters and accumulated cytokinesis defects could be observed at 104/ml. Although these cells exceed the 10-μm cutoff, we do not consider them to be true Titan cells because of these defects. In contrast, growth in minimal medium (YNB + 2% glucose) followed by low-density inoculation into 10% serum was sufficient to induce true Titan cells (Fig. 2a) [6••]. The impact of nutrients on induction was also reported by Hommell et al. and Trevijano-Contador et al., who note that pre-growth in either YPD or Sabouraud broth likewise inhibits induction of Titan-like cells [7••, 16••]. Interestingly, cells inoculated into a diluted minimal medium (5% Sabouraud buffered with MOPS and 5% FBS + 15 μM sodium azide) during induction produce proportionally sized daughter cells and also exhibit significant cytokinesis defects [7••]. Growth in Sabouraud has also been reported to inhibit the production of robust capsule, suggesting that nutrient availability is profoundly linked to the repression of virulence factor expression in this fungus [49].
Third, consistent with a role for nutrients and hypoxia in inducing Titanization, the cAMP/PKA pathway that mediates both of these signals is central to each of the in vitro protocols and is required for Titanization in vivo [6••, 7••, 16••, 18]. Elegant work from Choi et al. showed that perturbation of PKA1/PKR1 regulation is sufficient to generate spontaneous Titans in vitro [19]. Patient isolates with PKR1 truncations can be hyper-Titanizing in some instances, although the effect was dependent on genetic context [16••]. Despite this, activation of the cAMP/PKA pathway alone is not sufficient: pkr1Δ mutants do not constitutively form Titans, and the addition of exogenous dcAMP does not induce spontaneous Titanization [6••, 19].
How Does the cAMP Pathway Regulate Titanization?
The cAMP/PKA pathway is a central regulator of C. neoformans pathogenesis: cAMP/PKA regulates melanin production and capsule structure, and mutants deficient in this pathway are avirulent [50,51,52]. cAMP-deficient mutants are rapidly cleared from the lung, complicating efforts to study its role in virulence. Despite this, the influence of cAMP/PKA on Titanization was first described in vivo, building from the initial observation that Titan cell production increases upon coinfection with cells of opposite mating type [8]. Later, it was reported that the ste3α pheromone receptor and the G protein-coupled receptor Gpr5 are required for Titanization [18]. Both receptors interact with Gα protein Gpa1, which signals via the adenylyl cyclase Cac1 to generate cyclic AMP and relieve Pkr1-mediated negative regulation of Pka1 [50, 53]. Gpr5, Cac1, and Pka1 are required for in vitro Titan induction, and targets of this pathway, including the Rim101 and Gat201 transcription factors, also regulate Titanization [6••, 16••, 18].
Previous work has demonstrated that CnCac1 adenylyl cyclase activity is stimulated by bicarbonate and the β-carbonic anhydrase Can2, which is essential for growth in ambient CO2, but no activity has been previously attributed to CAN1 [54]. Consistent with cAMP/PKA regulation, loss of CAN1 increases the frequency of Titan-like cells [7••]. Although the specific mechanism underlying the can1Δ phenotype remains uncharacterized, oxygen tension influences Titanization in all three in vitro systems (Fig. 2) [6••, 7••, 16••].
This may speak to the intersection of cell cycle events and the yeast-to-Titan switch. It is well established that O2 limitation causes C. neoformans to decouple DNA synthesis and bud emergence [55, 56]. During hypoxic growth in rich medium, cells arrest in G2, post synthesis but prior to budding. Release into fresh rich medium triggers synchronized bud emergence for a single cell cycle, although this synchrony quickly collapses. In contrast, it appears that hypoxic growth in minimal medium prompts G1-S cycling in the absence of bud emergence (Fig. 1c), leading to the accumulation of a high percentage of Titan mothers [7••]. Subsequent release into rich or minimal medium enables bud emergence. Consistent with this, loss of the cell cycle-responsive transcription factor USV101 increases the frequency at which cells make the yeast-to-Titan switch [6••, 45]. We have recently reviewed progress on the interaction of the cell cycle with C. neoformans virulence and suggest that Titanization is similarly regulated at the level of the cell cycle [57]. A mechanistic understanding of how the above conditions influence these events is clearly needed.
Finally, accumulating evidence suggests a role for the mitochondrion in regulating fungal pathogenesis in general and the yeast-to-Titan switch in particular [58]. Perhaps most significant are the observations that perturbation of the mitochondrion, through the addition of the complex IV inhibitor sodium azide or through iron limitation, significantly increases the frequency of Titan-like cells [7••]. It is intriguing that the mitochondrion also influences capsule growth, another cAMP/PKA, and iron-regulated phenotype [59]. Moreover, transcription factors that influence capsule production (USV101, RIM101, GAT201) also influence Titanization [6••, 7••, 18, 45, 60]. Both RIM101 and GAT201 are negatively regulated by Usv101 and are targets of the cAMP/PKA pathway [45, 61]. In addition, the SAGA complex protein Ada2, which is a target of both cAMP and Gat201, is also required for both capsule and Titanization [7••, 60]. While the specific mechanisms by which these signals are integrated with mitochondrial function remain to be explored, these experiments are now within reach due to the development of in vitro Titanization assays.
How Do Host Dynamics Contribute to Titanization?
It is clear that the lung is particularly suited to Titan induction [6••, 8, 9]. From a microbial perspective, the lung is likely to be a hypoxic environment, particularly within fungal lesions [62]. However, host-relevant factors have also been shown to influence Titanization.
Host IgM levels have been implicated in in vivo Titan induction in the context of B cell inactivation [63]. T cell function has also been implicated: TH2-tilted C57BL/6J mice exhibit increased Titanization compared to TH1-tilted CD1 mice, and TH2 responses are associated with poor outcome [27, 32, 64]. Although the mechanisms driving fungal morphogenesis in these models remain unexplored, increased Titanization was associated with significant accumulation of the TH2-type cytokine IL4, increased eosinophilia, more abundant capsule IgM antibodies, and increased total IgE in sera [27].
Finally, the lung microbiome may influence Titanization: we have established that the bacterial cell wall component peptidoglycan is a key active component of serum sufficient for Titan cell induction [6••]. Healthy and diseased lungs are colonized by a robust bacterial microbiome [65, 66]. Upon inhalation of infectious spores or yeast, C. neoformans may sense this complex microbial environment and respond through a morphological transition from yeast to Titan form [6••]. The interaction of these microbial and host factors with fungal morphogenesis and drug resistance will have implications for disease progression and treatment.
How Do Titan Cells Contribute to Disease Progression?
Regardless of inducing condition, the yeast-to-Titan transition occurs within the first 24 h of exposure. In mouse models of infection, Titan cells can be observed in the lung 1 day post-infection and comprise up to 20% of fungal cells recovered through bronchio-alveolar lavage on day 3 [8]. When induced by mixing, or exposure to host-relevant ligands, Titan cells can be observed within the first 24 h, and overall size increases through continued culture.
Infection directly with Titan cells leads to increased lung fungal burdens early in infection [5].
In vivo, the overall median cell size decreases from 30 to 10 μm over the course of infection [67]. A similar phenomenon is observed during in vitro induction, with the relative proportion of small cells increasing more rapidly than the number of Titan cells over time [6••, 7••]. In addition to changes in cell size, Titans exhibited altered cell wall: chitin levels increase, while chitosan and other PAMP levels decrease, and this promotes a non-protective TH2 response [31, 32].
Up to 20% of patients may suffer from mixed infections with multiple serotypes, and there is evidence of the emergence of stable diploid serotype D or AD hybrid strains from patient isolates [17]. Sexual reproduction, through either bilateral or same-sex mating via the production of mono- or dikaryotic hyphae and the development of sporulating basidia, has never been observed in vivo for C. neoformans [68]. However, as mentioned above, Titan frequency increases during coinfection with strains of opposite mating type [8]. Both hybridization and Titanization lead to fundamental changes in cell ploidy that are likely to have significant implications for drug resistance. For example, 60% of patients will experience fluconazole resistance over the course of treatment [69]. Fluconazole resistance has been linked to the emergence of aneuploidy in chromosome 1, mediating increased gene dosage of the lanosterol 14α-demethylase ERG11 and drug exporter AFR1 [24]. While it is possible that exposure to fluconazole directly induces aneuploidy, as has been observed in vitro, the impact of Titanization on drug resistance in patients has not been investigated [70•]. However, it is clear that Titans exposed to fluconazole in vitro exhibit an increased frequency of aneuploidy and fluconazole resistance [14]. In addition, in vitro-induced Titan mothers produce aneuploid and diploid daughters at a high frequency even in the absence of chemical stress [6••]. Therefore, Titanization presents multiple possible mechanisms towards aneuploidy and drug resistance.
Concluding Remarks
With the development of robust in vitro induction protocols, the yeast-to-Titan transition has moved from an unusual host-specific virulence factor to a highly regulated morphological transition with profound implications for health and disease. Although many unanswered questions remain, we are now poised to investigate the basic biology of these cells to understand the molecular mechanisms underlying cryptococcal morphogenesis, drug resistance, and pathogenesis.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med. 2012;4(165):165rv13. https://doi.org/10.1126/scitranslmed.3004404.
Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS (London, England). 2009;23(4):525–30. https://doi.org/10.1097/QAD.0b013e328322ffac.
May RC, Stone NR, Wiesner DL, Bicanic T, Nielsen K. Cryptococcus: from environmental saprophyte to global pathogen. Nat Rev Microbiol. 2016;14(2):106–17. https://doi.org/10.1038/nrmicro.2015.6.
Denham ST, Brown JCS. Mechanisms of Pulmonary Escape and Dissemination by Cryptococcus neoformans. J Fungi (Basel). 2018;4(1) https://doi.org/10.3390/jof4010025.
Crabtree JN, Okagaki LH, Wiesner DL, Strain AK, Nielsen JN, Nielsen K. Titan cell production enhances the virulence of Cryptococcus neoformans. Infect Immun. 2012;80(11):3776–85. https://doi.org/10.1128/IAI.00507-12.
•• Dambuza IM, Drake T, Chapuis A, Zhou X, Correia J, Taylor-Smith L, et al. The Cryptococcus neoformans Titan cell is an inducible and regulated morphotype underlying pathogenesis. PLoS Pathogens. 2018;14(5):e1006978. https://doi.org/10.1371/journal.ppat.1006978. This paper describes in vitro Titan induction as shown in Fig. 2a.
•• Trevijano-Contador N, de Oliveira HC, García-Rodas R, Rossi SA, Llorente I, Zaballos Á, et al. Cryptococcus neoformans can form titan-like cells in vitro in response to multiple signals that require the activation of several transduction pathways. PLoS Pathog. 2018;14(5):e1007007. https://doi.org/10.1371/journal.ppat.1007007. This paper describes the in vitro induction of Titan-like cells as shown in Fig. 2a.
Okagaki LH, Strain AK, Nielsen JN, Charlier C, Baltes NJ, Chrétien F, et al. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog. 2010;6(6):e1000953. https://doi.org/10.1371/journal.ppat.1000953.
Zaragoza O, Garcia-Rivera J, Nosanchuk JD, Cuenca-Estrella M, Rodriguez-Tudela J, Casadevall A. Fungal cell gigantism during mammalian infection. PLoS Pathog. 2010;6(6):1–18.
Boyce KJ, Andrianopoulos A. Fungal dimorphism: the switch from hyphae to yeast is a specialized morphogenetic adaptation allowing colonization of a host. FEMS Microbiol Rev. 2015;39(6):797–811. https://doi.org/10.1093/femsre/fuv035.
Noble SM, Gianetti BA, Witchley JN. Candida albicans cell-type switching and functional plasticity in the mammalian host. Nat Rev Microbiol. 2017;15(2):96–108. https://doi.org/10.1038/nrmicro.2016.157.
Li Z, Nielsen K. Morphology changes in human fungal pathogens upon interaction with the host. J Fungi (Basel). 2017;3(4) https://doi.org/10.3390/jof3040066.
Hagen F, Khayhan K, Theelen B, Kolecka A, Polacheck I, Sionov E, et al. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet Biol: FG & B. 2015;78:16–48. https://doi.org/10.1016/j.fgb.2015.02.009.
• Gerstein AC, Fu MS, Mukaremera L, Li Z, Ormerod KL, Fraser JA, et al. Polyploid titan cells produce haploid and aneuploid progeny to promote stress adaptation. MBio. 2015;6(5):e01340-15. https://doi.org/10.1128/mBio.01340-15. This paper describes the influence of Titanization on the emergence of fluconazole resistance.
Zaragoza O, Nielsen K. Titan cells in Cryptococcus neoformans: cells with a giant impact. Curr Opin Microbiol. 2013;16(4):409–13. https://doi.org/10.1016/j.mib.2013.03.006.
•• Hommel B, Mukaremera L, Cordero RJB, Coelho C, Desjardins CA, Sturny-Leclere A, et al. Identification of environmental and genetic factors important for Cryptococcus neoformans titan cell formation using new in vitro inducing conditions. PLOS Pathogens. 2017;14(5):e1006982. https://doi.org/10.1101/191668. This paper describes the in vitro induction of Titan cells as shown in Fig. 2c.
Desnos-Ollivier M, Patel S, Spaulding AR, Charlier C, Garcia-Hermoso D, Nielsen K, et al. Mixed infections and In Vivo evolution in the human fungal pathogen Cryptococcus neoformans. MBio. 2010;1(1) https://doi.org/10.1128/mBio.00091-10.
Okagaki LH, Wang Y, Ballou ER, O'Meara TR, Bahn Y-S, Alspaugh JA, et al. Cryptococcal titan cell formation is regulated by G-protein signaling in response to multiple stimuli. Eukaryot Cell. 2011;10(10):1306–16. https://doi.org/10.1128/EC.05179-11.
Choi J, Vogl AW, Kronstad JW. Regulated expression of cyclic AMP-dependent protein kinase A reveals an influence on cell size and the secretion of virulence factors in Cryptococcus neoformans. Mol Microbiol. 2012;85(4):700–15. https://doi.org/10.1111/j.1365-2958.2012.08134.x.
Evans RJ, Li Z, Hughes WS, Djordjevic JT, Nielsen K, May RC. Cryptococcal phospholipase B1 is required for intracellular proliferation and control of titan cell morphology during macrophage infection. Infect Immun. 2015;83(4):1296–304. https://doi.org/10.1128/IAI.03104-14.
Chrisman CJ, Albuquerque P, Guimaraes AJ, Nieves E, Casadevall A. Phospholipids trigger Cryptococcus neoformans capsular enlargement during interactions with amoebae and macrophages. PLoS Pathog. 2011;7(5):e1002047. https://doi.org/10.1371/journal.ppat.1002047.
Cruickshank JG, Cavill R, Jelbert M. Cryptococcus neoformans of unusual morphology. Appl Microbiol. 1973;25(2):309–12.
Love GL, Boyd GD, Greer DL. Large Cryptococcus neoformans isolated from brain abscess. J Clin Microbiol. 1985;22(6):1068–70.
Sionov E, Chang YC, Garraffo HM, Kwon-Chung KJ. Heteroresistance to fluconazole in Cryptococcus neoformans is intrinsic and associated with virulence. Antimicrob Agents Chemother. 2009;53(7):2804–15. https://doi.org/10.1128/AAC.00295-09.
Hiruma M, Kagawa S. Ultrastructure of Cryptococcus neoformans in the cerebrospinal fluid of a patient with cryptococcal meningitis. Mycopathologia. 1985;89(1):5–12.
Robertson EJ, Najjuka G, Rolfes MA, Akampurira A, Jain N, Anantharanjit J, et al. Cryptococcus neoformans ex vivo capsule size is associated with intracranial pressure and host immune response in HIV-associated cryptococcal meningitis. J Infect Dis. 2014;209(1):74–82. https://doi.org/10.1093/infdis/jit435.
Garcia-Barbazan I, Trevijano-Contador N, Rueda C, de Andres B, Perez-Tavarez R, Herrero-Fernandez I, et al. The formation of titan cells in Cryptococcus neoformans depends on the mouse strain and correlates with induction of Th2-type responses. Cell Microbiol. 2016;18(1):111–24. https://doi.org/10.1111/cmi.12488.
Chen Y, Toffaletti DL, Tenor JL, Litvintseva AP, Fang C, Mitchell TG, et al. The Cryptococcus neoformans transcriptome at the site of human meningitis. MBio. 2014;5(1):e01087–13. https://doi.org/10.1128/mBio.01087-13.
Brinker T, Stopa E, Morrison J, Klinge P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS. 2014;11:10. https://doi.org/10.1186/2045-8118-11-10.
van Leeuwen LM, Evans RJ, Jim KK, Verboom T, Fang X, Bojarczuk A, et al. A transgenic zebrafish model for the in vivo study of the blood and choroid plexus brain barriers using claudin 5. Biol Open. 2018;7(2) https://doi.org/10.1242/bio.030494.
Mukaremera L, Lee KK, Wagener J, Wiesner DL, Gow NAR, Nielsen K. Titan cell production in Cryptococcus neoformans reshapes the cell wall and capsule composition during infection. Cell Surface. 2018;1(1):15–24. https://doi.org/10.1016/j.tcsw.2017.12.001.
Wiesner DL, Specht CA, Lee CK, Smith KD, Mukaremera L, Lee ST, et al. Chitin recognition via chitotriosidase promotes pathologic type-2 helper T cell responses to cryptococcal infection. PLoS Pathog. 2015;11(3):e1004701. https://doi.org/10.1371/journal.ppat.1004701.
Baddley JW, Forrest GN. Cryptococcosis in solid organ transplantation. Am J Trans. 13(s4):242–9. https://doi.org/10.1111/ajt.12116.
Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis. 2017;17(8):873–81. https://doi.org/10.1016/S1473-3099(17)30243-8.
Singh N, Alexander BD, Lortholary O, Dromer F, Gupta KL, John GT, et al. Cryptococcus neoformans in organ transplant recipients: impact of calcineurin-inhibitor agents on mortality. J Infect Dis. 2007;195(5):756–64. https://doi.org/10.1086/511438.
Williamson PR, Jarvis JN, Panackal AA, Fisher MC, Molloy SF, Loyse A, et al. Cryptococcal meningitis: epidemiology, immunology, diagnosis and therapy. Nat Rev Neurol. 2016;13:13–24. https://doi.org/10.1038/nrneurol.2016.167.
Chau T, Mai N, Phu N, Nghia H, Chuong L, Sinh D et al. A prospective descriptive study of cryptococcal meningitis in HIV uninfected patients in Vietnam - high prevalence of Cryptococcus neoformans var grubii in the absence of underlying disease. 2010:1–8.
Lomes NR, Melhem MS, Szeszs MW, Martins Mdos A, Buccheri R. Cryptococcosis in non-HIV/non-transplant patients: a Brazilian case series. Med Mycol. 2016;54(7):669–76. https://doi.org/10.1093/mmy/myw021.
Raval K, Powderly W, Spec A. Presentation and outcome of cryptococcal infection varies by predisposing illness. Open Forum Infect Dis. 2017;4(suppl_1):S77–S8. https://doi.org/10.1093/ofid/ofx163.016.
Wong ML, Back P, Candy G, Nelson G, Murray J. Cryptococcal pneumonia in African miners at autopsy. Int J Tuberc Lung Dis. 2007;11(5):528–33.
Scriven JE, Botha FC, Schutz C, Lalloo DG, Wainwright H, Meintjes G. Paradoxical respiratory failure due to cryptococcal pneumonia after amphotericin B treatment for HIV-associated cryptococcal meningitis. Med Mycol Case Rep. 2018;2018(19):38–40. https://doi.org/10.1016/j.mmcr.2017.02.004.
Bielska E, May RC. What makes Cryptococcus gattii a pathogen? FEMS Yeast Res. 2016;16(1):fov106. doi:https://doi.org/10.1093/femsyr/fov106.
Shirley RM, Baddley JW. Cryptococcal lung disease. Curr Opin Pulm Med. 2009;15(3):254–60. https://doi.org/10.1097/MCP.0b013e328329268d.
Okagaki LH, Nielsen K. Titan cells confer protection from phagocytosis in Cryptococcus neoformans infections. Eukaryot Cell. 2012;11(6):820–6. https://doi.org/10.1128/EC.00121-12.
Gish SR, Maier EJ, Haynes BC, Santiago-Tirado FH, Srikanta DL, Ma CZ, et al. Computational analysis reveals a key regulator of cryptococcal virulence and determinant of host response. MBio. 2016;7(2):e00313–6. https://doi.org/10.1128/mBio.00313-16.
Vanhove M, Beale MA, Rhodes J, Chanda D, Lakhi S, Kwenda G, et al. Genomic epidemiology of Cryptococcus yeasts identifies adaptation to environmental niches underpinning infection across an African HIV/AIDS cohort. Mol Ecol. 2016;26:1991–2005. https://doi.org/10.1111/mec.13891.
Homer CM, Summers DK, Goranov AI, Clarke SC, Wiesner DL, Diedrich JK, et al. Intracellular action of a secreted peptide required for fungal virulence. Cell Host Microbe. 2016;19(6):849–64. https://doi.org/10.1016/j.chom.2016.05.001.
Albuquerque P, Nicola AM, Nieves E, Paes HC, Williamson PR, Silva-Pereira I, et al. Quorum sensing-mediated, cell density-dependent regulation of growth and virulence in Cryptococcus neoformans. MBio. 2013;5(1):e00986–13. https://doi.org/10.1128/mBio.00986-13.
Zaragoza O, Fries BC, Casadevall A. Induction of capsule growth in Cryptococcus neoformans by mammalian serum and CO(2). Infect Immun. 2003;71(11):6155–64.
Alspaugh JA, Pukkila-Worley R, Harashima T, Cavallo LM, Funnell D, Cox GM, et al. Adenylyl cyclase functions downstream of the Galpha protein Gpa1 and controls mating and pathogenicity of Cryptococcus neoformans. Eukaryot Cell. 2002;1(1):75–84.
D'Souza CA, Alspaugh JA, Yue C, Harashima T, Cox GM, Perfect JR, et al. Cyclic AMP-dependent protein kinase controls virulence of the fungal pathogen Cryptococcus neoformans. Mol Cell Biol. 2001;21(9):3179–91. https://doi.org/10.1128/MCB.21.9.3179-3191.2001.
Alspaugh JA, Perfect JR, Heitman J. Cryptococcus neoformans mating and virulence are regulated by the G-protein alpha subunit GPA1 and cAMP. Genes Dev. 1997;11(23):3206–17.
Xue C, Bahn Y-S, Cox GM, Heitman J. G protein-coupled receptor Gpr4 senses amino acids and activates the cAMP-PKA pathway in Cryptococcus neoformans. Mol Biol Cell. 2006;17(2):667–79. https://doi.org/10.1091/mbc.E05-07-0699.
Mogensen EG, Janbon G, Chaloupka J, Steegborn C, Fu MS, Moyrand F, et al. Cryptococcus neoformans senses CO2 through the carbonic anhydrase Can2 and the adenylyl cyclase Cac1. Eukaryot Cell. 2006;5(1):103–11. https://doi.org/10.1128/EC.5.1.103-111.2006.
Ohkusu M, Raclavsky V, Takeo K. Deficit in oxygen causes G2 budding and unbudded G2 arrest in Cryptococcus neoformans. FEMS Microbiol Lett. 2001;204:29–32.
Yamaguchi M, Ohkusu M, Biswas SK, Kawamoto S. Cytological study of cell cycle of the pathogenic yeast Cryptococcus neoformans. Nihon Ishinkin Gakkai zasshi = Japanese journal of medical mycology. 2007;48(4):147–52.
Ballou ER, Johnston SA. The cause and effect of Cryptococcus interactions with the host. Curr Opin Microbiol. 2017;40:88–94. https://doi.org/10.1016/j.mib.2017.10.012.
Warris A, Ballou ER. Oxidative responses and fungal infection biology. Semin Cell Dev Biol. 2018; https://doi.org/10.1016/j.semcdb.2018.03.004.
Trevijano-Contador N, Rossi SA, Alves E, Landin-Ferreiroa S, Zaragoza O. Capsule enlargement in Cryptococcus neoformans is dependent on mitochondrial activity. Front Microbiol. 2017;8:1423. https://doi.org/10.3389/fmicb.2017.01423.
Haynes BC, Skowyra ML, Spencer SJ, Gish SR, Williams M, Held EP, et al. Toward an integrated model of capsule regulation in Cryptococcus neoformans. PLoS Pathog. 2011;7(12):e1002411. https://doi.org/10.1371/journal.ppat.1002411.
O'Meara TR, Norton D, Price MS, Hay C, Clements MF, Nichols CB, et al. Interaction of Cryptococcus neoformans Rim101 and protein kinase a regulates capsule. PLoS Pathog. 2010;6(2):e1000776. https://doi.org/10.1371/journal.ppat.1000776.
Grahl N, Puttikamonkul S, Macdonald JM, Gamcsik MP, Ngo LY, Hohl TM, et al. In vivo hypoxia and a fungal alcohol dehydrogenase influence the pathogenesis of invasive pulmonary aspergillosis. PLoS Pathog. 2011;7(7):e1002145. https://doi.org/10.1371/journal.ppat.1002145.
Szymczak WA, Davis MJ, Lundy SK, Dufaud C, Olszewski M, Pirofski LA. X-linked immunodeficient mice exhibit enhanced susceptibility to Cryptococcus neoformans infection. MBio. 2013;4(4) https://doi.org/10.1128/mBio.00265-13.
Hernandez Y, Arora S, Erb-Downward JR, McDonald RA, Toews GB, Huffnagle GB. Distinct roles for IL-4 and IL-10 in regulating T2 immunity during allergic bronchopulmonary mycosis. J Immunol. 2005;174(2):1027–36.
Erb-Downward JR, Thompson DL, Han MK, Freeman CM, McCloskey L, Schmidt LA, et al. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS One. 2011;6(2):e16384. https://doi.org/10.1371/journal.pone.0016384.
Dickson RP, Huffnagle GB. The lung microbiome: new principles for respiratory bacteriology in health and disease. PLoS Pathog. 2015;11(7):e1004923. https://doi.org/10.1371/journal.ppat.1004923.
Denham ST, Verma S, Reynolds RC, Worne CL, Daugherty JM, Lane TE, et al. Regulated release of cryptococcal polysaccharide drives virulence and suppresses immune infiltration into the central nervous system. Infect Immun. 2017;86 https://doi.org/10.1128/IAI.00662-17.
Wang L, Zhai B, Lin X. The link between morphotype transition and virulence in Cryptococcus neoformans. PLoS Pathog. 2012;8(6):e1002765. https://doi.org/10.1371/journal.ppat.1002765.
Bicanic T, Harrison T, Niepieklo A, Dyakopu N, Meintjes G. Symptomatic relapse of HIV-associated cryptococcal meningitis after initial fluconazole monotherapy: the role of fluconazole resistance and immune reconstitution. Clin Infect Dis. 2006;43(8):1069–73. https://doi.org/10.1086/507895.
• Altamirano S, Fang D, Simmons C, Sridhar S, Wu P, Sanyal K, et al. Fluconazole-induced ploidy change in Cryptococcus neoformans results from the uncoupling of cell growth and nuclear division. mSphere. 2017;2(3) https://doi.org/10.1128/mSphere.00205-17. This work proposes a mechanism for the emergence of fluconazole resistance in Cryptococcus neoformans.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Mycology
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zhou, X., Ballou, E.R. The Cryptococcus neoformans Titan Cell: From In Vivo Phenomenon to In Vitro Model. Curr Clin Micro Rpt 5, 252–260 (2018). https://doi.org/10.1007/s40588-018-0107-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40588-018-0107-9