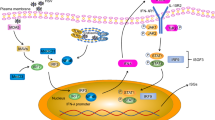
Abstract
Purpose of Review
The earliest host-virus interactions occur during virus attachment and entry into cells. These initial steps in the virus lifecycle influence the outcome of infection beyond delivery of the viral genome into the cell. Herpesviruses alter host signaling pathways and processes during attachment and entry to facilitate virus infection and modulate innate immune responses. We suggest in this review that understanding these early events may inform the rational design of therapeutic and prevention strategies for herpesvirus infection, as well as the engineering of viral vectors for immunotherapy purposes.
Recent Findings
Recent reports demonstrate that modulation of herpes simplex virus type-1 (HSV-1) entry results in unexpected enhancement of anti-viral immune responses.
Summary
A variety of evidence suggests that herpesviruses promote specific cellular signaling responses that facilitate viral replication after binding to cell surfaces, as well as during virus entry. Of particular interest is the ability of the virus to alter innate immune responses through these cellular signaling events. Uncovering the underlying immune evasion strategies may lead to the design of live-attenuated vaccines that can generate robust and protective anti-viral immune responses against herpesviruses. These adjuvant properties may be extended to a variety of heterologous antigens expressed by herpesviral vectors.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Roizman B, Knipe D, Whitley RJ. Herpes simplex viruses. In: HP KDM, editor. Fields Virology. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2013. p. 1823–97.
Looker KJ, Magaret AS, May MT, Turner KM, Vickerman P, Gottlieb SL, et al. Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS One. 2015;10(10):e0140765. https://doi.org/10.1371/journal.pone.0140765.
Looker KJ, Magaret AS, Turner KM, Vickerman P, Gottlieb SL, Newman LM. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS One. 2015;10(1):e114989. https://doi.org/10.1371/journal.pone.0114989.
Pottage JC Jr, Kessler HA. Herpes simplex virus resistance to acyclovir: clinical relevance. Infect Agents Dis. 1995;4(3):115–24.
Castelo-Soccio L, Bernardin R, Stern J, Goldstein SA, Kovarik C. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch Dermatol. 2010;146(2):124–6. https://doi.org/10.1001/archdermatol.2009.363.
Kemble G, Spaete R. Herpes simplex vaccines. In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, et al., editors. Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge: Cambridge University Press; 2007. http://www.ncbi.nlm.nih.gov/pubmed/21348132.
Stanfield B, Kousoulas KG. Herpes simplex vaccines: prospects of live-attenuated HSV vaccines to combat genital and ocular infections. Curr Clin Microbiol Rep. 2015;2(3):125–36. https://doi.org/10.1007/s40588-015-0020-4.
Gianni T, Leoni V, Chesnokova LS, Hutt-Fletcher LM, Campadelli-Fiume G. Alphavbeta3-integrin is a major sensor and activator of innate immunity to herpes simplex virus-1. Proc Natl Acad Sci U S A. 2012;109(48):19792–7. https://doi.org/10.1073/pnas.1212597109.
Paludan SR, Bowie AG, Horan KA, Fitzgerald KA. Recognition of herpesviruses by the innate immune system. Nat Rev Immunol. 2011;11(2):143–54. https://doi.org/10.1038/nri2937.
Casiraghi C, Gianni T, Campadelli-Fiume G. Alphavbeta3 integrin boosts the innate immune response elicited in epithelial cells through plasma membrane and endosomal toll-like receptors. J Virol. 2016;90(8):4243–8. https://doi.org/10.1128/JVI.03175-15.
Cai M, Li M, Wang K, Wang S, Lu Q, Yan J, et al. The herpes simplex virus 1-encoded envelope glycoprotein B activates NF-kappaB through the toll-like receptor 2 and MyD88/TRAF6-dependent signaling pathway. PLoS One. 2013;8(1):e54586. https://doi.org/10.1371/journal.pone.0054586.
Kim M, Osborne NR, Zeng W, Donaghy H, McKinnon K, Jackson DC, et al. Herpes simplex virus antigens directly activate NK cells via TLR2, thus facilitating their presentation to CD4 T lymphocytes. J Immunol. 2012;188(9):4158–70. https://doi.org/10.4049/jimmunol.1103450.
Holm CK, Jensen SB, Jakobsen MR, Cheshenko N, Horan KA, Moeller HB, et al. Virus-cell fusion as a trigger of innate immunity dependent on the adaptor STING. Nat Immunol. 2012;13(8):737–43. https://doi.org/10.1038/ni.2350.
Stevenson EV, Collins-McMillen D, Kim JH, Cieply SJ, Bentz GL, Yurochko AD. HCMV reprogramming of infected monocyte survival and differentiation: a goldilocks phenomenon. Viruses. 2014;6(2):782–807. https://doi.org/10.3390/v6020782.
Yurochko AD. Human cytomegalovirus modulation of signal transduction. Curr Top Microbiol Immunol. 2008;325:205–20.
Kurt-Jones EA, Orzalli MH, Knipe DM. Innate immune mechanisms and herpes simplex virus infection and disease. Adv Anat Embryol Cell Biol. 2017;223:49–75. https://doi.org/10.1007/978-3-319-53168-7_3.
McCormick AL, Mocarski ES Jr. Viral modulation of the host response to infection. In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, et al., editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press; 2007. http://www.ncbi.nlm.nih.gov/pubmed/21348102.
Medici MA, Sciortino MT, Perri D, Amici C, Avitabile E, Ciotti M, et al. Protection by herpes simplex virus glycoprotein D against Fas-mediated apoptosis: role of nuclear factor kappaB. J Biol Chem. 2003;278(38):36059–67. https://doi.org/10.1074/jbc.M306198200.
Liu X, Fitzgerald K, Kurt-Jones E, Finberg R, Knipe DM. Herpesvirus tegument protein activates NF-kappaB signaling through the TRAF6 adaptor protein. Proc Natl Acad Sci U S A. 2008;105(32):11335–9. https://doi.org/10.1073/pnas.0801617105.
Campadelli-Fiume G, Collins-McMillen D, Gianni T, Yurochko AD. Integrins as herpesvirus receptors and mediators of the host signalosome. Annu Rev Virol. 2016;3(1):215–36. https://doi.org/10.1146/annurev-virology-110615-035618.
Chan G, Nogalski MT, Stevenson EV, Yurochko AD. Human cytomegalovirus induction of a unique signalsome during viral entry into monocytes mediates distinct functional changes: a strategy for viral dissemination. J Leukoc Biol. 2012;92(4):743–52. https://doi.org/10.1189/jlb.0112040.
Azab W, Osterrieder K. Initial contact: the first steps in herpesvirus entry. Adv Anat Embryol Cell Biol. 2017;223:1–27. https://doi.org/10.1007/978-3-319-53168-7_1.
Eisenberg RJ, Atanasiu D, Cairns TM, Gallagher JR, Krummenacher C, Cohen GH. Herpes virus fusion and entry: a story with many characters. Viruses. 2012;4(5):800–32. https://doi.org/10.3390/v4050800.
Heldwein EE, Krummenacher C. Entry of herpesviruses into mammalian cells. Cell Mol Life Sci. 2008;65(11):1653–68. https://doi.org/10.1007/s00018-008-7570-z.
Turner A, Bruun B, Minson T, Browne H. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J Virol. 1998;72(1):873–5.
Ruyechan WT, Morse LS, Knipe DM, Roizman B. Molecular genetics of herpes simplex virus. II. Mapping of the major viral glycoproteins and of the genetic loci specifying the social behavior of infected cells. J Virol. 1979;29(2):677–97.
Dolter KE, Ramaswamy R, Holland TC. Syncytial mutations in the herpes simplex virus type 1 gK (UL53) gene occur in two distinct domains. J Virol. 1994;68(12):8277–81.
Bond VC, Person S, Warner SC. The isolation and characterization of mutants of herpes simplex virus type 1 that induce cell fusion. J Gen Virol. 1982;61(Pt 2):245–54. https://doi.org/10.1099/0022-1317-61-2-245.
Read GS, Person S, Keller PM. Genetic studies of cell fusion induced by herpes simplex virus type 1. J Virol. 1980;35(1):105–13.
Pogue-Geile KL, Spear PG. The single base pair substitution responsible for the Syn phenotype of herpes simplex virus type 1, strain MP. Virology. 1987;157(1):67–74. https://doi.org/10.1016/0042-6822(87)90314-X.
Avitabile E, Lombardi G, Gianni T, Capri M, Campadelli-Fiume G. Coexpression of UL20p and gK inhibits cell-cell fusion mediated by herpes simplex virus glycoproteins gD, gH-gL, and wild-type gB or an endocytosis-defective gB mutant and downmodulates their cell surface expression. J Virol. 2004;78(15):8015–25. https://doi.org/10.1128/JVI.78.15.8015-8025.2004.
Agelidis AM, Shukla D. Cell entry mechanisms of HSV: what we have learned in recent years. Futur Virol. 2015;10(10):1145–54. https://doi.org/10.2217/fvl.15.85.
Nicola AV, Hou J, Major EO, Straus SE. Herpes simplex virus type 1 enters human epidermal keratinocytes, but not neurons, via a pH-dependent endocytic pathway. J Virol. 2005;79(12):7609–16. https://doi.org/10.1128/JVI.79.12.7609-7616.2005.
Clement C, Tiwari V, Scanlan PM, Valyi-Nagy T, Yue BY, Shukla D. A novel role for phagocytosis-like uptake in herpes simplex virus entry. J Cell Biol. 2006;174(7):1009–21. https://doi.org/10.1083/jcb.200509155.
Gianni T, Salvioli S, Chesnokova LS, Hutt-Fletcher LM, Campadelli-Fiume G. Alphavbeta6- and alphavbeta8-integrins serve as interchangeable receptors for HSV gH/gL to promote endocytosis and activation of membrane fusion. PLoS Pathog. 2013;9(12):e1003806. https://doi.org/10.1371/journal.ppat.1003806.
Nicola AV, Straus SE. Cellular and viral requirements for rapid endocytic entry of herpes simplex virus. J Virol. 2004;78(14):7508–17. https://doi.org/10.1128/JVI.78.14.7508-7517.2004.
Nicola AV. Herpesvirus entry into host cells mediated by endosomal low pH. Traffic. 2016;17(9):965–75. https://doi.org/10.1111/tra.12408.
Azab W, Gramatica A, Herrmann A, Osterrieder N. Binding of alphaherpesvirus glycoprotein H to surface alpha4beta1-integrins activates calcium-signaling pathways and induces phosphatidylserine exposure on the plasma membrane. MBio. 2015;6(5):e01552–15. https://doi.org/10.1128/mBio.01552-15.
Ryckman BJ, Jarvis MA, Drummond DD, Nelson JA, Johnson DC. Human cytomegalovirus entry into epithelial and endothelial cells depends on genes UL128 to UL150 and occurs by endocytosis and low-pH fusion. J Virol. 2006;80(2):710–22. https://doi.org/10.1128/JVI.80.2.710-722.2006.
Archer MA, Brechtel TM, Davis LE, Parmar RC, Hasan MH, Tandon R. Inhibition of endocytic pathways impacts cytomegalovirus maturation. Sci Rep. 2017;7:46069. https://doi.org/10.1038/srep46069.
Oxford KL, Strelow L, Yue Y, Chang WL, Schmidt KA, Diamond DJ, et al. Open reading frames carried on UL/b' are implicated in shedding and horizontal transmission of rhesus cytomegalovirus in rhesus monkeys. J Virol. 2011;85(10):5105–14. https://doi.org/10.1128/JVI.02631-10.
Spear PG. Herpes simplex virus: receptors and ligands for cell entry. Cell Microbiol. 2004;6(5):401–10. https://doi.org/10.1111/j.1462-5822.2004.00389.x.
Shukla D, Liu J, Blaiklock P, Shworak NW, Bai X, Esko JD, et al. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell. 1999;99(1):13–22. https://doi.org/10.1016/S0092-8674(00)80058-6.
Montgomery RI, Warner MS, Lum BJ, Spear PG. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87(3):427–36. https://doi.org/10.1016/S0092-8674(00)81363-X.
Cocchi F, Menotti L, Mirandola P, Lopez M, Campadelli-Fiume G. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J Virol. 1998;72(12):9992–10002.
Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280(5369):1618–20. https://doi.org/10.1126/science.280.5369.1618.
Satoh T, Arii J, Suenaga T, Wang J, Kogure A, Uehori J, et al. PILRalpha is a herpes simplex virus-1 entry coreceptor that associates with glycoprotein B. Cell. 2008;132(6):935–44. https://doi.org/10.1016/j.cell.2008.01.043.
Arii J, Goto H, Suenaga T, Oyama M, Kozuka-Hata H, Imai T, et al. Non-muscle myosin IIA is a functional entry receptor for herpes simplex virus-1. Nature. 2010;467(7317):859–62. https://doi.org/10.1038/nature09420.
Suenaga T, Satoh T, Somboonthum P, Kawaguchi Y, Mori Y, Arase H. Myelin-associated glycoprotein mediates membrane fusion and entry of neurotropic herpesviruses. Proc Natl Acad Sci U S A. 2010;107(2):866–71. https://doi.org/10.1073/pnas.0913351107.
Taylor JM, Lin E, Susmarski N, Yoon M, Zago A, Ware CF, et al. Alternative entry receptors for herpes simplex virus and their roles in disease. Cell Host Microbe. 2007;2(1):19–28. https://doi.org/10.1016/j.chom.2007.06.005.
Di Giovine P, Settembre EC, Bhargava AK, Luftig MA, Lou H, Cohen GH, et al. Structure of herpes simplex virus glycoprotein D bound to the human receptor nectin-1. PLoS Pathog. 2011;7(9):e1002277. https://doi.org/10.1371/journal.ppat.1002277.
Carfi A, Willis SH, Whitbeck JC, Krummenacher C, Cohen GH, Eisenberg RJ, et al. Herpes simplex virus glycoprotein D bound to the human receptor HveA. Mol Cell. 2001;8(1):169–79. https://doi.org/10.1016/S1097-2765(01)00298-2.
Cheshenko N, Liu W, Satlin LM, Herold BC. Multiple receptor interactions trigger release of membrane and intracellular calcium stores critical for herpes simplex virus entry. Mol Biol Cell. 2007;18(8):3119–30. https://doi.org/10.1091/mbc.E07-01-0062.
Shui JW, Kronenberg M. HVEM: an unusual TNF receptor family member important for mucosal innate immune responses to microbes. Gut Microbes. 2013;4(2):146–51. https://doi.org/10.4161/gmic.23443.
Steinberg MW, Cheung TC, Ware CF. The signaling networks of the herpesvirus entry mediator (TNFRSF14) in immune regulation. Immunol Rev. 2011;244(1):169–87. https://doi.org/10.1111/j.1600-065X.2011.01064.x.
Murphy TL, Murphy KM. Slow down and survive: enigmatic immunoregulation by BTLA and HVEM. Annu Rev Immunol. 2010;28(1):389–411. https://doi.org/10.1146/annurev-immunol-030409-101202.
Cheung TC, Steinberg MW, Oborne LM, Macauley MG, Fukuyama S, Sanjo H, et al. Unconventional ligand activation of herpesvirus entry mediator signals cell survival. Proc Natl Acad Sci U S A. 2009;106(15):6244–9. https://doi.org/10.1073/pnas.0902115106.
Lasaro MO, Tatsis N, Hensley SE, Whitbeck JC, Lin SW, Rux JJ, et al. Targeting of antigen to the herpesvirus entry mediator augments primary adaptive immune responses. Nat Med. 2008;14(2):205–12. https://doi.org/10.1038/nm1704.
Sciortino MT, Medici MA, Marino-Merlo F, Zaccaria D, Giuffre-Cuculletto M, Venuti A, et al. Involvement of gD/HVEM interaction in NF-kB-dependent inhibition of apoptosis by HSV-1 gD. Biochem Pharmacol. 2008;76(11):1522–32. https://doi.org/10.1016/j.bcp.2008.07.030.
Edwards RG, Longnecker R. Herpesvirus entry mediator and ocular herpesvirus infection: more than meets the eye. J Virol. 2017;91(13):e00115–7. https://doi.org/10.1128/JVI.00115-17.
Karaba AH, Kopp SJ, Longnecker R. Herpesvirus entry mediator is a serotype specific determinant of pathogenesis in ocular herpes. Proc Natl Acad Sci U S A. 2012;109(50):20649–54. https://doi.org/10.1073/pnas.1216967109.
Karaba AH, Kopp SJ, Longnecker R. Herpesvirus entry mediator and nectin-1 mediate herpes simplex virus 1 infection of the murine cornea. J Virol. 2011;85(19):10041–7. https://doi.org/10.1128/JVI.05445-11.
Feire AL, Roy RM, Manley K, Compton T. The glycoprotein B disintegrin-like domain binds beta 1 integrin to mediate cytomegalovirus entry. J Virol. 2010;84(19):10026–37. https://doi.org/10.1128/JVI.00710-10.
Garrigues HJ, Rubinchikova YE, Dipersio CM, Rose TM. Integrin alphaVbeta3 binds to the RGD motif of glycoprotein B of Kaposi’s sarcoma-associated herpesvirus and functions as an RGD-dependent entry receptor. J Virol. 2008;82(3):1570–80. https://doi.org/10.1128/JVI.01673-07.
Leoni V, Gianni T, Salvioli S, Campadelli-Fiume G. Herpes simplex virus glycoproteins gH/gL and gB bind Toll-like receptor 2, and soluble gH/gL is sufficient to activate NF-kappaB. J Virol. 2012;86(12):6555–62. https://doi.org/10.1128/JVI.00295-12.
Fournier N, Chalus L, Durand I, Garcia E, Pin JJ, Churakova T, et al. FDF03, a novel inhibitory receptor of the immunoglobulin superfamily, is expressed by human dendritic and myeloid cells. J Immunol. 2000;165(3):1197–209. https://doi.org/10.4049/jimmunol.165.3.1197.
Wang J, Shiratori I, Uehori J, Ikawa M, Arase H. Neutrophil infiltration during inflammation is regulated by PILRalpha via modulation of integrin activation. Nat Immunol. 2013;14(1):34–40. https://doi.org/10.1038/ni.2456.
Parry C, Bell S, Minson T, Browne H. Herpes simplex virus type 1 glycoprotein H binds to alphavbeta3 integrins. J Gen Virol. 2005;86(Pt 1):7–10. https://doi.org/10.1099/vir.0.80567-0.
Cheshenko N, Trepanier JB, Gonzalez PA, Eugenin EA, Jacobs WR Jr, Herold BC. Herpes simplex virus type 2 glycoprotein H interacts with integrin alphavbeta3 to facilitate viral entry and calcium signaling in human genital tract epithelial cells. J Virol. 2014;88(17):10026–38. https://doi.org/10.1128/JVI.00725-14.
Cheshenko N, Trepanier JB, Stefanidou M, Buckley N, Gonzalez P, Jacobs W, et al. HSV activates Akt to trigger calcium release and promote viral entry: novel candidate target for treatment and suppression. FASEB J. 2013;27(7):2584–99. https://doi.org/10.1096/fj.12-220285.
Cheshenko N, Del Rosario B, Woda C, Marcellino D, Satlin LM, Herold BC. Herpes simplex virus triggers activation of calcium-signaling pathways. J Cell Biol. 2003;163(2):283–93. https://doi.org/10.1083/jcb.200301084.
Gianni T, Massaro R, Campadelli-Fiume G. Dissociation of HSV gL from gH by alphavbeta6- or alphavbeta8-integrin promotes gH activation and virus entry. Proc Natl Acad Sci U S A. 2015;112(29):E3901–10. https://doi.org/10.1073/pnas.1506846112.
Gianni T, Campadelli-Fiume G. The epithelial alphavbeta3-integrin boosts the MYD88-dependent TLR2 signaling in response to viral and bacterial components. PLoS Pathog. 2014;10(11):e1004477. https://doi.org/10.1371/journal.ppat.1004477.
Gianni T, Campadelli-Fiume G. AlphaVbeta3-integrin relocalizes nectin1 and routes herpes simplex virus to lipid rafts. J Virol. 2012;86(5):2850–5. https://doi.org/10.1128/JVI.06689-11.
Iversen MB, Reinert LS, Thomsen MK, Bagdonaite I, Nandakumar R, Cheshenko N, et al. An innate antiviral pathway acting before interferons at epithelial surfaces. Nat Immunol. 2016;17(2):150–8. https://doi.org/10.1038/ni.3319.
Tiwari V, Shukla D. Phosphoinositide 3 kinase signalling may affect multiple steps during herpes simplex virus type-1 entry. J Gen Virol. 2010;91(Pt 12):3002–9. https://doi.org/10.1099/vir.0.024166-0.
MacLeod IJ, Minson T. Binding of herpes simplex virus type-1 virions leads to the induction of intracellular signalling in the absence of virus entry. PLoS One. 2010;5(3):e9560. https://doi.org/10.1371/journal.pone.0009560.
Liu X, Cohen JI. The role of PI3K/Akt in human herpesvirus infection: from the bench to the bedside. Virology. 2015;479-480:568–77. https://doi.org/10.1016/j.virol.2015.02.040.
Zheng K, Xiang Y, Wang X, Wang Q, Zhong M, Wang S, et al. Epidermal growth factor receptor-PI3K signaling controls cofilin activity to facilitate herpes simplex virus 1 entry into neuronal cells. MBio. 2014;5(1):e00958–13. https://doi.org/10.1128/mBio.00958-13.
Mostowy S, Shenoy AR. The cytoskeleton in cell-autonomous immunity: structural determinants of host defence. Nat Rev Immunol. 2015;15(9):559–73. https://doi.org/10.1038/nri3877.
Fukazawa A, Alonso C, Kurachi K, Gupta S, Lesser CF, McCormick BA, et al. GEF-H1 mediated control of NOD1 dependent NF-kappaB activation by Shigella effectors. PLoS Pathog. 2008;4(11):e1000228. https://doi.org/10.1371/journal.ppat.1000228.
Keestra AM, Winter MG, Auburger JJ, Frassle SP, Xavier MN, Winter SE, et al. Manipulation of small rho GTPases is a pathogen-induced process detected by NOD1. Nature. 2013;496(7444):233–7. https://doi.org/10.1038/nature12025.
Dunn W, Chou C, Li H, Hai R, Patterson D, Stolc V, et al. Functional profiling of a human cytomegalovirus genome. Proc Natl Acad Sci U S A. 2003;100(24):14223–8. https://doi.org/10.1073/pnas.2334032100.
Wang D, Shenk T. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc Natl Acad Sci U S A. 2005;102(50):18153–8. https://doi.org/10.1073/pnas.0509201102.
Hai R, Chu A, Li H, Umamoto S, Rider P, Liu F. Infection of human cytomegalovirus in cultured human gingival tissue. Virol J. 2006;3(1):84. https://doi.org/10.1186/1743-422X-3-84.
Borza CM, Hutt-Fletcher LM. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat Med. 2002;8(6):594–9. https://doi.org/10.1038/nm0602-594.
Jambunathan N, Charles AS, Subramanian R, Saied AA, Naderi M, Rider P, et al. Deletion of a predicted beta-sheet domain within the amino terminus of herpes simplex virus glycoprotein K conserved among alphaherpesviruses prevents virus entry into neuronal axons. J Virol. 2015;90(5):2230–9. https://doi.org/10.1128/JVI.02468-15.
Li G, Nguyen CC, Ryckman BJ, Britt WJ, Kamil JPA. Viral regulator of glycoprotein complexes contributes to human cytomegalovirus cell tropism. Proc Natl Acad Sci U S A. 2015;112(14):4471–6. https://doi.org/10.1073/pnas.1419875112.
Li G, Kamil JP. Viral regulation of cell tropism in human cytomegalovirus. J Virol. 2015;90(2):626–9. https://doi.org/10.1128/JVI.01500-15.
Mori Y. Recent topics related to human herpesvirus 6 cell tropism. Cell Microbiol. 2009;11(7):1001–6. https://doi.org/10.1111/j.1462-5822.2009.01312.x.
Hutt-Fletcher LM, Lake CM. Two Epstein-Barr virus glycoprotein complexes. Curr Top Microbiol Immunol. 2001;258:51–64.
Sathiyamoorthy K, Hu YX, Mohl BS, Chen J, Longnecker R, Jardetzky TS. Structural basis for Epstein-Barr virus host cell tropism mediated by gp42 and gHgL entry glycoproteins. Nat Commun. 2016;7:13557. https://doi.org/10.1038/ncomms13557.
Saied AA, Chouljenko VN, Subramanian R, Kousoulas KGA. Replication competent HSV-1(McKrae) with a mutation in the amino-terminus of glycoprotein K (gK) is unable to infect mouse trigeminal ganglia after cornea infection. Curr Eye Res. 2014;39(6):596–603. https://doi.org/10.3109/02713683.2013.855238.
Chouljenko VN, Iyer AV, Chowdhury S, Kim J, Kousoulas KG. The herpes simplex virus type 1 UL20 protein and the amino terminus of glycoprotein K (gK) physically interact with gB. J Virol. 2010;84(17):8596–606. https://doi.org/10.1128/JVI.00298-10.
Chowdhury S, Chouljenko VN, Naderi M, Kousoulas KG. The amino terminus of herpes simplex virus 1 glycoprotein K is required for virion entry via the paired immunoglobulin-like type-2 receptor alpha. J Virol. 2013;87(6):3305–13. https://doi.org/10.1128/JVI.02982-12.
Wang K, Kappel JD, Canders C, Davila WF, Sayre D, Chavez M, et al. A herpes simplex virus 2 glycoprotein D mutant generated by bacterial artificial chromosome mutagenesis is severely impaired for infecting neuronal cells and infects only Vero cells expressing exogenous HVEM. J Virol. 2012;86(23):12891–902. https://doi.org/10.1128/JVI.01055-12.
Hansen SG, Sacha JB, Hughes CM, Ford JC, Burwitz BJ, Scholz I, et al. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science. 2013;340(6135):1237874. https://doi.org/10.1126/science.1237874.
Barouch DH, Picker LJ. Novel vaccine vectors for HIV-1. Nat Rev Microbiol. 2014;12(11):765–71. https://doi.org/10.1038/nrmicro3360.
Wussow F, Yue Y, Martinez J, Deere JD, Longmate J, Herrmann A, et al. A vaccine based on the rhesus cytomegalovirus UL128 complex induces broadly neutralizing antibodies in rhesus macaques. J Virol. 2013;87(3):1322–32. https://doi.org/10.1128/JVI.01669-12.
Wussow F, Chiuppesi F, Martinez J, Campo J, Johnson E, Flechsig C, et al. Human cytomegalovirus vaccine based on the envelope gH/gL pentamer complex. PLoS Pathog. 2014;10(11):e1004524. https://doi.org/10.1371/journal.ppat.1004524.
Zhou G, Roizman B. Construction and properties of a herpes simplex virus 1 designed to enter cells solely via the IL-13alpha2 receptor. Proc Natl Acad Sci U S A. 2006;103(14):5508–13. https://doi.org/10.1073/pnas.0601258103.
Zhou G, Ye GJ, Debinski W, Roizman B. Engineered herpes simplex virus 1 is dependent on IL13Ralpha 2 receptor for cell entry and independent of glycoprotein D receptor interaction. Proc Natl Acad Sci U S A. 2002;99(23):15124–9. https://doi.org/10.1073/pnas.232588699.
Campadelli-Fiume G, Petrovic B, Leoni V, Gianni T, Avitabile E, Casiraghi C, et al. Retargeting strategies for oncolytic herpes simplex viruses. Viruses. 2016;8(3):63. https://doi.org/10.3390/v8030063.
Gambini E, Reisoli E, Appolloni I, Gatta V, Campadelli-Fiume G, Menotti L, et al. Replication-competent herpes simplex virus retargeted to HER2 as therapy for high-grade glioma. Mol Ther. 2012;20(5):994–1001. https://doi.org/10.1038/mt.2012.22.
Nanni P, Gatta V, Menotti L, De Giovanni C, Ianzano M, Palladini A, et al. Preclinical therapy of disseminated HER-2(+) ovarian and breast carcinomas with a HER-2-retargeted oncolytic herpesvirus. PLoS Pathog. 2013;9(1):e1003155. https://doi.org/10.1371/journal.ppat.1003155.
Gatta V, Petrovic B, Campadelli-Fiume G. The engineering of a novel ligand in gH confers to HSV an expanded tropism independent of gD activation by its receptors. PLoS Pathog. 2015;11(5):e1004907. https://doi.org/10.1371/journal.ppat.1004907.
Leoni V, Gatta V, Palladini A, Nicoletti G, Ranieri D, Dall'Ora M, et al. Systemic delivery of HER2-retargeted oncolytic-HSV by mesenchymal stromal cells protects from lung and brain metastases. Oncotarget. 2015;6(33):34774–87. https://doi.org/10.18632/oncotarget.5793.
Leoni V, Gatta V, Casiraghi C, Nicosia A, Petrovic B, Campadelli-Fiume GA. Strategy for cultivation of retargeted oncolytic herpes simplex viruses in non-cancer cells. J Virol. 2017;91(10):e00067–17. https://doi.org/10.1128/JVI.00067-17.
Petrovic B, Gianni T, Gatta V, Campadelli-Fiume G. Insertion of a ligand to HER2 in gB retargets HSV tropism and obviates the need for activation of the other entry glycoproteins. PLoS Pathog. 2017;13(4):e1006352. https://doi.org/10.1371/journal.ppat.1006352.
Petro CD, Weinrick B, Khajoueinejad N, Burn C, Sellers R, Jacobs WR Jr, et al. HSV-2 DeltagD elicits FcgammaR-effector antibodies that protect against clinical isolates. JCI Insight. 2016;1(12) https://doi.org/10.1172/jci.insight.88529.
Boukhvalova M, McKay J, Mbaye A, Sanford-Crane H, Blanco JC, Huber A, et al. Efficacy of the herpes simplex virus 2 (HSV-2) glycoprotein D/AS04 vaccine against genital HSV-2 and HSV-1 infection and disease in the cotton rat Sigmodon Hispidus model. J Virol. 2015;89(19):9825–40. https://doi.org/10.1128/JVI.01387-15.
• Petro C, Gonzalez PA, Cheshenko N, Jandl T, Khajoueinejad N, Benard A, et al. Herpes simplex type 2 virus deleted in glycoprotein D protects against vaginal, skin and neural disease. elife. 2015;4:e06054. This manuscript demonstrates the efficaciousness of a gD deficient HSV-2 vaccine is due to ADCC.
Royer DJ, Carr MM, Gurung HR, Halford WP, Carr DJJ. The neonatal fc receptor and complement fixation facilitate prophylactic vaccine-mediated humoral protection against viral infection in the ocular mucosa. J Immunol. 2017;199(5):1898–911. https://doi.org/10.4049/jimmunol.1700316.
• Wang K, Goodman KN, Li DY, Raffeld M, Chavez M, Cohen JI. A herpes simplex virus 2 (HSV-2) gD mutant impaired for neural tropism is superior to an HSV-2 gD subunit vaccine to protect animals from challenge with HSV-2. J Virol. 2015;90(1):562–574. This report demonstrates the utility of a stable mutation in HSV-2 abrogating binding of gD to nectin-1 provides protection of mice from lethal HSV-2 challenge. https://doi.org/10.1128/JVI.01845-15.
MacLean CA, Efstathiou S, Elliott ML, Jamieson FE, McGeoch DJ. Investigation of herpes simplex virus type 1 genes encoding multiply inserted membrane proteins. J Gen Virol. 1991;72(Pt 4):897–906. https://doi.org/10.1099/0022-1317-72-4-897.
Melancon JM, Foster TP, Kousoulas KG. Genetic analysis of the herpes simplex virus type 1 (HSV-1) UL20 protein domains involved in cytoplasmic virion envelopment and virus-induced cell fusion. J Virol. 2004;78(14):7329–43. https://doi.org/10.1128/JVI.78.14.7329-7343.2004.
Baines JD, Ward PL, Campadelli-Fiume G, Roizman B. The UL20 gene of herpes simplex virus 1 encodes a function necessary for viral egress. J Virol. 1991;65(12):6414–24.
Bzik DJ, Fox BA, DeLuca NA, Person S. Nucleotide sequence of a region of the herpes simplex virus type 1 gB glycoprotein gene: mutations affecting rate of virus entry and cell fusion. Virology. 1984;137(1):185–90. https://doi.org/10.1016/0042-6822(84)90022-9.
Pellett PE, Kousoulas KG, Pereira L, Roizman B. Anatomy of the herpes simplex virus 1 strain F glycoprotein B gene: primary sequence and predicted protein structure of the wild type and of monoclonal antibody-resistant mutants. J Virol. 1985;53(1):243–53.
Bond VC, Person S. Fine structure physical map locations of alterations that affect cell fusion in herpes simplex virus type 1. Virology. 1984;132(2):368–76. https://doi.org/10.1016/0042-6822(84)90042-4.
Debroy C, Pederson N, Person S. Nucleotide sequence of a herpes simplex virus type 1 gene that causes cell fusion. Virology. 1985;145(1):36–48. https://doi.org/10.1016/0042-6822(85)90199-0.
Hutchinson L, Goldsmith K, Snoddy D, Ghosh H, Graham FL, Johnson DC. Identification and characterization of a novel herpes simplex virus glycoprotein, gK, involved in cell fusion. J Virol. 1992;66(9):5603–9.
Pogue-Geile KL, Lee GT, Shapira SK, Spear PG. Fine mapping of mutations in the fusion-inducing MP strain of herpes simplex virus type 1. Virology. 1984;136(1):100–9. https://doi.org/10.1016/0042-6822(84)90251-4.
Kim IJ, Chouljenko VN, Walker JD, Kousoulas KG. Herpes simplex virus 1 glycoprotein M and the membrane-associated protein UL11 are required for virus-induced cell fusion and efficient virus entry. J Virol. 2013;87(14):8029–37. https://doi.org/10.1128/JVI.01181-13.
Foster TP, Chouljenko VN, Kousoulas KG. Functional and physical interactions of the herpes simplex virus type 1 UL20 membrane protein with glycoprotein K. J Virol. 2008;82(13):6310–23. https://doi.org/10.1128/JVI.00147-08.
Foster TP, Melancon JM, Baines JD, Kousoulas KG. The herpes simplex virus type 1 UL20 protein modulates membrane fusion events during cytoplasmic virion morphogenesis and virus-induced cell fusion. J Virol. 2004;78(10):5347–57. https://doi.org/10.1128/JVI.78.10.5347-5357.2004.
Melancon JM, Luna RE, Foster TP, Kousoulas KG. Herpes simplex virus type 1 gK is required for gB-mediated virus-induced cell fusion, while neither gB and gK nor gB and UL20p function redundantly in virion de-envelopment. J Virol. 2005;79(1):299–313. https://doi.org/10.1128/JVI.79.1.299-313.2005.
Rider PJF, Naderi M, Bergeron S, Chouljenko VN, Brylinski M, Kousoulas KG. Cysteines and N-glycosylation sites conserved among all alphaherpesviruses regulate membrane fusion in herpes simplex virus type 1 infection. J Virol. 2017;91(21):e00873–17. https://doi.org/10.1128/JVI.00873-17.
• Stanfield BA, Stahl J, Chouljenko VN, Subramanian R, Charles AS, Saied AA, et al. A single intramuscular vaccination of mice with the HSV-1 VC2 virus with mutations in the glycoprotein K and the membrane protein UL20 confers full protection against lethal intravaginal challenge with virulent HSV-1 and HSV-2 strains. PLoS One. 2014;9(10):e109890. This manuscript demonstrates efficacy of an HSV-1 vaccine which possesses a mutation in gK that affects neuronal tropism against both HSV-1 and HSV-2 challenge in a mouse model. https://doi.org/10.1371/journal.pone.0109890.
Stanfield BA, Pahar B, Chouljenko VN, Veazey R, Kousoulas KG. Vaccination of rhesus macaques with the live-attenuated HSV-1 vaccine VC2 stimulates the proliferation of mucosal T cells and germinal center responses resulting in sustained production of highly neutralizing antibodies. Vaccine. 2017;35(4):536–43. https://doi.org/10.1016/j.vaccine.2016.12.018.
Musarrat F, Jambunathan N, Rider PJF, Chouljenko VN, Kousoulas KG. The Amino-terminus of HSV-1 Glycoprotein K (gK) is Required for gB Binding to Akt, Release of Intracellular Calcium and Fusion of the Viral Envelope with Plasma Membranes. J Virol. 2018. https://doi.org/10.1128/JVI.01842-17.
Murphy JE, Padilla BE, Hasdemir B, Cottrell GS, Bunnett NW. Endosomes: a legitimate platform for the signaling train. Proc Natl Acad Sci U S A. 2009;106(42):17615–22. https://doi.org/10.1073/pnas.0906541106.
Gleeson PA. The role of endosomes in innate and adaptive immunity. Semin Cell Dev Biol. 2014;31:64–72. https://doi.org/10.1016/j.semcdb.2014.03.002.
Brubaker SW, Bonham KS, Zanoni I, Kagan JC. Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol. 2015;33(1):257–90. https://doi.org/10.1146/annurev-immunol-032414-112240.
Acknowledgements
We regret that the important works of many of our colleagues were outside the scope of this review and were thus unable to be discussed. We would like to thank Rhonda Cardin for the comments and critical reading of the manuscript. The work was supported by the LSU Division of Biotechnology and Molecular Medicine, by a Governor’s Biotechnology Initiative Grant (KGK) and Cores of the Center for Experimental Infectious Disease Research (CEIDR) supported by the NIH:NIGMS Grant P30GM110670.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Kousoulas declares a pending patent for vaccines against genital herpes simplex infections (United States Patent Application 20170266275 pending).
Paul J.F. Rider, Farhana Musarrat, Rafiq Nabi, and Shan Naidu declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Recent Developments in Anti-viral Vaccines
Rights and permissions
About this article
Cite this article
Rider, P.J.F., Musarrat, F., Nabi, R. et al. First Impressions—the Potential of Altering Initial Host-Virus Interactions for Rational Design of Herpesvirus Vaccine Vectors. Curr Clin Micro Rpt 5, 55–65 (2018). https://doi.org/10.1007/s40588-018-0082-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40588-018-0082-1