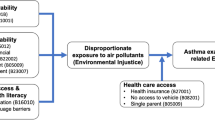
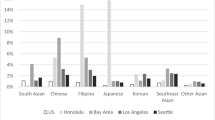
Abstract
Purpose of Review
Neurotoxicant exposures are of particular concern in historically marginalized communities. Often a consequence of structural racism, low-income minoritized populations experience a disproportionate burden of hazardous exposures through proximity to industrial facilities, high traffic roads, and suboptimal housing. Here, we summarize reports on exposures and neurodevelopment focused on differences by education, income, race/ethnicity, or immigration status from 2015 to 2022, discuss the importance of such investigations in overburdened communities, and recommend areas for future research.
Recent Findings
We found 20 studies that investigated exposure disparities and neurodevelopment in children. Most were conducted in the USA, and many focused on air pollution, followed by metal exposures and water contamination. Although several studies showed differences in exposure-outcome associations by income and education, many examining differences by race/ethnicity did not report notable disparities between groups. However, measures of individual race and ethnicity are not reliable measures of discrimination experienced as a consequence of structural racism.
Summary
Our review supports scientific evidence that the reduction of individual and widespread municipal exposures will improve child development and overall public health. Identified research gaps include the use of better indicators of economic status and structural racism, evaluations of effect modification and attributable fraction of outcomes by these factors, and considerations of multidimensional neighborhood factors that could be protective against environmental insults. Considering that vulnerable populations have disparities in access to and quality of care, greater burden of exposure, and fewer resources to incur associated expenses, such populations should be prioritized.



Similar content being viewed by others
Data Availability
Data used for this review have been provided by the authors in a supplemental file.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ismail FY, Shapiro BK. What are neurodevelopmental disorders? Curr Opin Neurol. 2019;32(4):611–16.
Carlsson T, Molander F, Taylor MJ, Jonsson U, Bölte S. Early environmental risk factors for neurodevelopmental disorders - a systematic review of twin and sibling studies. Dev Psychopathol. 2021;33(4):1448–95.
Gaylord A, Osborne G, Ghassabian A, Malits J, Attina T, Trasande L. Trends in neurodevelopmental disability burden due to early life chemical exposure in the USA from 2001 to 2016: a population-based disease burden and cost analysis. Mol Cell Endocrinol. 2020;502:110666.
Lyall K, Croen L, Daniels J, Fallin MD, Ladd-Acosta C, Lee BK, et al. The changing epidemiology of autism spectrum disorders. Annu Rev Public Health. 2017;38:81–102.
Kalkbrenner AE, Schmidt RJ, Penlesky AC. Environmental chemical exposures and autism spectrum disorders: a review of the epidemiological evidence. Curr Probl Pediatr Adolesc Health Care. 2014;44(10):277–318.
Koehler K, Latshaw M, Matte T, Kass D, Frumkin H, Fox M, et al. Building healthy community environments: a public health approach. Public Health Rep. 2018;133(1_suppl):35s–43s.
Thapar A, Rutter M. Genetic Advances in Autism. J Autism Dev Disord. 2021;51(12):4321–32.
Su JG, Jerrett M, Morello-Frosch R, Jesdale BM, Kyle AD. Inequalities in cumulative environmental burdens among three urbanized counties in California. Environ Int. 2012;40:79–87.
Morello-Frosch R, Shenassa ED. The environmental “riskscape” and social inequality: implications for explaining maternal and child health disparities. Environ Health Perspect. 2006;114(8):1150–3.
Morello-Frosch R, Zuk M, Jerrett M, Shamasunder B, Kyle AD. Understanding the cumulative impacts of inequalities in environmental health: implications for policy. Health Affairs (Project Hope). 2011;30(5):879–87.
Payne-Sturges DC, Gee GC, Cory-Slechta DA. Confronting racism in environmental health sciences: moving the science forward for eliminating racial inequities. Environ Health Perspect. 2021;129(5):55002. Provides commentary on the importance of studying environmental exposure disparities in historically marginalized groups.
Hahler E-M, Elsabbagh M. Autism: a global perspective. Curr Dev Disord Rep. 2015;2(1):58–64.
Zeidan J, Fombonne E, Scorah J, Ibrahim A, Durkin MS, Saxena S, et al. Global prevalence of autism: a systematic review update. Autism Res. 2022;15(5):778–90.
Mustapha A, Amegah AK, Coker ES. Harmonization of epidemiologic research methods to address the environmental and social determinants of urban slum health challenges in Sub-Saharan Africa. Int J Environ Res Public Health. 2022;19(18):11273. Provides a commentary on the importance and difficulty of studying environmental justice issues in low- and middle-income countries.
Bullard RD. Sacrifice zones: the front lines of toxic chemical exposure in the United States. Environ Health Perspect. 2011;119(6):A266-A.
Armstrong R, Hall BJ, Doyle J, Waters E. Cochrane Update. ‘Scoping the scope’ of a cochrane review. J Public Health (Oxf). 2011;33(1):147–50.
Babineau J. Product review: covidence (systematic review software). J Can Health Libr Assoc / J de l’Association des bibliothèques de la santé du Can. 2014;35(2):68–71.
Mullen C, Grineski SE, Collins TW, Mendoza DL. Effects of PM(2.5) on third grade students’ proficiency in math and english language arts. Int J Environ Res Public Health. 2020;17(18):6931.
Grineski SE, Clark-Reyna SE, Collins TW. School-based exposure to hazardous air pollutants and grade point average: a multi-level study. Environ Res. 2016;147:164–71.
Braun JM, Hornung R, Chen A, Dietrich KN, Jacobs DE, Jones R, et al. Effect of residential lead-hazard interventions on childhood blood lead concentrations and neurobehavioral outcomes: a randomized clinical trial. JAMA Pediatr. 2018;172(10):934–42.
Frndak S, Barg G, Canfield RL, Quierolo EI, Mañay N, Kordas K. Latent subgroups of cognitive performance in lead- and manganese-exposed Uruguayan children: examining behavioral signatures. Neurotoxicology. 2019;73:188–98.
Sears L, Myers JV, Sears CG, Brock GN, Zhang C, Zierold KM. Manganese body burden in children is associated with reduced visual motor and attention skills. Neurotoxicol Teratol. 2021;88:107021.
Haynes EN, Sucharew H, Hilbert TJ, Kuhnell P, Spencer A, Newman NC, et al. Impact of air manganese on child neurodevelopment in East Liverpool. Ohio Neurotoxicology. 2018;64:94–102.
Sarigiannis DA, Karakitsios SP. Addressing complexity of health impact assessment in industrially contaminated sites via the exposome paradigm. Epidemiol Prev. 2018;42(5–6s1):37–48.
Lucchini RG, Guazzetti S, Renzetti S, Conversano M, Cagna G, Fedrighi C, et al. Neurocognitive impact of metal exposure and social stressors among schoolchildren in Taranto, Italy. Environ Health. 2019;18(1):67.
McGuinn LA, Wiggins LD, Volk HE, Di Q, Moody EJ, Kasten E, et al. Pre- and postnatal fine particulate matter exposure and childhood cognitive and adaptive function. Int J Environ Res Public Health. 2022;19(7):3748. One of the most recent manuscripts to use prospectively collected cohort data to evaluated differences in air pollutant exposures and cognitive risk by race and maternal education.
Araya F, Stingone JA, Claudio L. Inequalities in exposure to ambient air neurotoxicants and disparities in markers of neurodevelopment in children by maternal nativity status. Int J Environ Res Public Health. 2021;18(14):7512.
Nazif-Muñoz JI, Spengler JD, Arku RE, Oulhote Y. Solid fuel use and early child development disparities in Ghana: analyses by gender and urbanicity. J Eposure Sci Environ Epidemiol. 2020;30(4):698–706.
Rana J, Luna-Gutiérrez P, Haque SE, Ignacio Nazif-Muñoz J, Mitra DK, Oulhote Y. Associations between household air pollution and early child development among children aged 36–59 months in Bangladesh. J Epidemiol Community Health. 2022;76(7):667–76. This study uses investigate indoor air pollution in a populations within a highly exposure low- and middle-income country.
Emerson E, Savage A, Llewellyn G. Significant cognitive delay among 3- to 4-year old children in low- and middle-income countries: prevalence estimates and potential impact of preventative interventions. Int J Epidemiol. 2018;47(5):1465–74.
Sania A, Sudfeld CR, Danaei G, Fink G, McCoy DC, Zhu Z, et al. Early life risk factors of motor, cognitive and language development: a pooled analysis of studies from low/middle-income countries. BMJ Open. 2019;9(10):e026449.
Vishnevetsky J, Tang D, Chang HW, Roen EL, Wang Y, Rauh V, et al. Combined effects of prenatal polycyclic aromatic hydrocarbons and material hardship on child IQ. Neurotoxicol Teratol. 2015;49:74–80.
Pearson AL, Shewark EA, Burt SA. Associations between neighborhood built, social, or toxicant conditions and child externalizing behaviors in the Detroit metro area: a cross-sectional study of the neighborhood ‘exposome.’ BMC Public Health. 2022;22(1):1064. This recent publication investigates how joint environmental exposures and social stressor interact in relation to child behaviors in a particularly vulnerable population.
Dellefratte K, Stingone JA, Claudio L. Combined association of BTEX and material hardship on ADHD-suggestive behaviours among a nationally representative sample of US children. Paediatr Perinat Epidemiol. 2019;33(6):482–9.
Dumcke TS, Benedetti A, Selistre LDS, Camardelo AMP, Silva ERD. Association between exposure to urban waste and emotional and behavioral difficulties in schoolchildren. J Pediatr. 2020;96(3):364–70.
Dickerson AS, Rahbar MH, Pearson DA, Kirby RS, Bakian AV, Bilder DA, et al. Autism spectrum disorder reporting in lower socioeconomic neighborhoods. Autism. 2017;21(4):470–80.
Miller HL, Thomi M, Patterson RM, Nandy K. Effects of intersectionality along the pathway to diagnosis for autistic children with and without co-occurring attention deficit hyperactivity disorder in a nationally-representative sample. J Autism Dev Disord. 2022.
Dickerson AS, Dickerson AS. Prenatal socioenvironmental exposures and autism spectrum disorder: a web of confusion. Child Dev Perspect. 2022;17(1):32–8. Provide a conceptual framework to support the purpose of this review and a discussion around how joint environmental exposures and psychosocial stressors impact neurodevelopment.
Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13(3):330–8.
Lam J, Sutton P, Kalkbrenner A, Windham G, Halladay A, Koustas E, et al. A systematic review and meta-analysis of multiple airborne pollutants and autism spectrum disorder. PLoS one. 2016;11(9):e0161851.
Castagna A, Mascheroni E, Fustinoni S, Montirosso R. Air pollution and neurodevelopmental skills in preschool- and school-aged children: a systematic review. Neurosci Biobehav Rev. 2022;136:104623.
Engelhardt B, Liebner S. Novel insights into the development and maintenance of the blood-brain barrier. Cell Tissue Res. 2014;355(3):687–99.
Kovacs CS. Maternal mineral and bone metabolism during pregnancy, lactation, and post-weaning recovery. Physiol Rev. 2016;96(2):449–547.
Schug TT, Blawas AM, Gray K, Heindel JJ, Lawler CP. Elucidating the links between endocrine disruptors and neurodevelopment. Endocrinology. 2015;156(6):1941–51.
Attina TM, Malits J, Naidu M, Trasande L. Racial/ethnic disparities in disease burden and costs related to exposure to endocrine-disrupting chemicals in the United States: an exploratory analysis. J Clin Epidemiol. 2019;108:34–43.
Perera FP, Wheelock K, Wang Y, Tang D, Margolis AE, Badia G, et al. Combined effects of prenatal exposure to polycyclic aromatic hydrocarbons and material hardship on child ADHD behavior problems. Environ Res. 2018;160:506–13.
Gavino-Lopez N, Eaves LA, Enggasser AE, Fry RC. Developing toxic metal environmental justice indices (TM-EJIs) for arsenic, cadmium, lead, and manganese contamination in private drinking wells in North Carolina. Water (Basel). 2022;14(13):2088.
Burns J, Angelino AC, Lewis K, Gotcsik ME, Bell RA, Bell J, et al. Land rights and health outcomes in American Indian/Alaska native children. Pediatrics. 2021;148(5):e2020041350.
Sobel M, Sanchez TR, Zacher T, Mailloux B, Powers M, Yracheta J, et al. Spatial relationship between well water arsenic and uranium in Northern Plains native lands. Environ Pollut. 2021;287:117655.
DeFur PL, Evans GW, Cohen Hubal EA, Kyle AD, Morello-Frosch RA, Williams DR. Vulnerability as a function of individual and group resources in cumulative risk assessment. Environ Health Perspect. 2007;115(5):817–24.
Mikati I, Benson AF, Luben TJ, Sacks JD, Richmond-Bryant J. Disparities in distribution of particulate matter emission sources by race and poverty status. Am J Public Health. 2018;108(4):480–5.
Arcaya MC, Tucker-Seeley RD, Kim R, Schnake-Mahl A, So M, Subramanian SV. Research on neighborhood effects on health in the United States: a systematic review of study characteristics. Soc Sci Med. 2016;168:16–29.
Smith GS, Breakstone H, Dean LT, Thorpe RJ Jr. Impacts of gentrification on health in the US: a systematic review of the literature. J Urban Health. 2020;97(6):845–56.
Dean LT, Nicholas LH. Using credit scores to understand predictors and consequences of disease. Am J Public Health. 2018;108(11):1503–5.
Dean LT, Thorpe RJ. What structural racism is (or is not) and how to measure it: clarity for public health and medical researchers. Am J Epidemiol. 2022;191(9):1521–6.
Shonkoff JP, Garner AS. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1):e232–46.
Smith GS, Anjum E, Francis C, Deanes L, Acey C. Climate change, environmental disasters, and health inequities: the underlying role of structural inequalities. Curr Environ Health Rep. 2022;9(1):80–9. Discusses policies, community mechanisms, and structural inequities that lead to environmental exposure disparities and justice issues in minoritized communities.
Tuck E. Suspending damage: a letter to communities. Harv Educ Rev. 2009;79(3):409–28.
Theriault N, Kang S. Toxic research: political ecologies and the matter of damage. Environ Soc. 2021;12(1):5–24.
Caron RM, Tshabangu-Soko T. Environmental inequality: childhood lead poisoning as an inadvertent consequence of the refugee resettlement process. J Progress Hum Serv. 2012;23(3):208–22.
Bax AC, Bard DE, Cuffe SP, McKeown RE, Wolraich ML. The association between race/ethnicity and socioeconomic factors and the diagnosis and treatment of children with attention-deficit hyperactivity disorder. J Dev Behav Pediatr. 2019;40(2):81–91.
Bishop-Fitzpatrick L, Kind AJH. A scoping review of health disparities in autism spectrum disorder. J Autism Dev Disord. 2017;47(11):3380–91.
Nasol E, Lindly OJ, Chavez AE, Zuckerman KE. Unmet need and financial impact disparities for US children with ADHD. Acad Pediatr. 2019;19(3):315–24.
Acknowledgements
We thank Lori Rosman, lead informationist at the Welch Medical Library, for her assistance in selecting our search terms and compiling all the references for our literature review.
Funding
This work was supported by the Hopkins Center for Health Disparities Solutions (U54MD000214). Aisha Dickerson is partially supported by funds from the National Institute of Environmental Health Science (K01ES032046: PI, Dickerson), an Opportunities and Infrastructure Fund (OIF) sub-award via NIH funding to the Environmental Influence on Child Health Outcomes (ECHO) program (U2COD023375-06), and Bloomberg Philanthropies through the Bloomberg American Health Initiative at the Johns Hopkins Bloomberg School of Public Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Early Life Environmental Health
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dickerson, A.S., Frndak, S., DeSantiago, M. et al. Environmental Exposure Disparities and Neurodevelopmental Risk: a Review. Curr Envir Health Rpt 10, 73–83 (2023). https://doi.org/10.1007/s40572-023-00396-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40572-023-00396-6