Abstract
Purpose of Review
This review summarizes inorganic arsenic (iAs) metabolism and toxicity in mice and the gut microbiome and how iAs and the gut microbiome interact to induce diseases.
Recent Findings
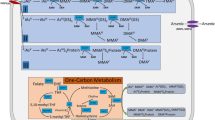
Recently, a variety of studies have started to reveal the interactions between iAs and the gut microbiome. Evidence shows that gut bacteria can influence iAs biotransformation and disease risks. The gut microbiome can directly metabolize iAs, and it can also indirectly be involved in iAs metabolism through the host, such as altering iAs absorption, cofactors, and genes related to iAs metabolism. Many factors, such as iAs metabolism influenced by the gut microbiome, and microbiome metabolites perturbed by iAs can lead to different disease risks.
Summary
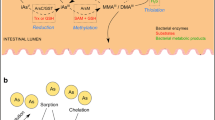
iAs is a widespread toxic metalloid in environment, and iAs toxicity has become a global health issue. iAs is subject to metabolic reactions after entering the host body, including methylation, demethylation, oxidation, reduction, and thiolation. Different arsenic species, including trivalent and pentavalent forms and inorganic and organic forms, determine their toxicity. iAs poisoning is predominately caused by contaminated drinking water and food, and chronic arsenic toxicity can cause various diseases. Therefore, studies of iAs metabolism are important for understanding iAs associated disease risks.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Humans, I.W.G.o.t.E.o.C.R.t., W.H. Organization, and I.A.f.R.o. Cancer. Some drinking-water disinfectants and contaminants, including arsenic, vol. 84. Lyon: IARC; 2004.
Nordstrom DK. Worldwide occurrences of arsenic in ground water. In: American Association for the Advancement of Science; 2002.
Authority EFS. Dietary exposure to inorganic arsenic in the European population. EFSA J. 2014;12(3):3597.
Taylor V, et al. Human exposure to organic arsenic species from seafood. Sci Total Environ. 2017;580:266–82.
Vahter M, Concha G. Role of metabolism in arsenic toxicity. Pharmacol Toxicol. 2001;89(1):1–5.
Cohen SM, et al. Evaluation of the carcinogenicity of inorganic arsenic. Crit Rev Toxicol. 2013;43(9):711–52.
Vahter M. Metabolism of arsenic. Biol Environ Effects Ars. 1983;6:171–98.
Kapaj S, et al. Human health effects from chronic arsenic poisoning–a review. J Environ Sci Health A. 2006;41(10):2399–428.
Kuo C-C, et al. The association of arsenic metabolism with cancer, cardiovascular disease, and diabetes: a systematic review of the epidemiological evidence. Environ Health Perspect. 2017;125(8):087001.
Cheng T-J, Ke D-S, Guo H-R. The association between arsenic exposure from drinking water and cerebrovascular disease mortality in Taiwan. Water Res. 2010;44(19):5770–6.
Rahman M, et al. A prospective cohort study of stroke mortality and arsenic in drinking water in Bangladeshi adults. BMC Public Health. 2014;14(1):174.
Watanabe T, Hirano S. Metabolism of arsenic and its toxicological relevance. Arch Toxicol. 2013;87(6):969–79.
Hirano S, et al. Difference in uptake and toxicity of trivalent and pentavalent inorganic arsenic in rat heart microvessel endothelial cells. Arch Toxicol. 2003;77(6):305–12.
Healy SM, et al. Enzymatic methylation of arsenic compounds: V. arsenite methyltransferase activity in tissues of mice. Toxicol Appl Pharmacol. 1998;148(1):65–70.
Uhlon M, Fagerberg L, Hallstrom B. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419.
Liu Z, et al. Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proc Natl Acad Sci. 2002;99(9):6053–8.
Meng Y-L, Liu Z, Rosen BP. As (III) and Sb (III) uptake by GlpF and efflux by ArsB in Escherichia coli. J Biol Chem. 2004;279(18):18334–41.
Rosenberg H, Gerdes R, Chegwidden K. Two systems for the uptake of phosphate in Escherichia coli. J Bacteriol. 1977;131(2):505–11.
Cullen WR. Chemical Mechanism of Arsenic Biomethylation. Chem Res Toxicol. 2014;27(4):457–61.
Challenger F. Biological methylation. Chem Rev. 1945;36(3):315–61.
Dheeman DS, et al. Pathway of human AS3MT arsenic methylation. Chem Res Toxicol. 2014;27(11):1979–89 This article proposed a new and detailed pathway of arsenic methylation and briefly summarized Challenger pathway and Hayakawa pathway.
Hayakawa T, et al. A new metabolic pathway of arsenite: arsenic–glutathione complexes are substrates for human arsenic methyltransferase Cyt19. Arch Toxicol. 2005;79(4):183–91.
Lin S, et al. A NovelS-adenosyl-L-methionine: arsenic (III) methyltransferase from rat liver cytosol. J Biol Chem. 2002;277(13):10795–803.
Chowdhury UK, et al. Glutathione-S-transferase-omega [MMA (V) reductase] knockout mice: enzyme and arsenic species concentrations in tissues after arsenate administration. Toxicol Appl Pharmacol. 2006;216(3):446–57.
Radabaugh TR, et al. Arsenate reductase II. Purine nucleoside phosphorylase in the presence of dihydrolipoic acid Is a route for reduction of arsenate to arsenite in mammalian systems. Chem Res Toxicol. 2002;15(5):692–8.
Naranmandura H, et al. Arsenic metabolism and thioarsenicals in hamsters and rats. Chem Res Toxicol. 2007;20(4):616–24.
Hansen HR, et al. Sulfur-containing arsenical mistaken for dimethylarsinous acid [DMA (III)] and identified as a natural metabolite in urine: major implications for studies on arsenic metabolism and toxicity. Chem Res Toxicol. 2004;17(8):1086–91.
Raab A, et al. Pentavalent arsenic can bind to biomolecules. Angew Chem Int Ed. 2007;46(15):2594–7.
Fan C, et al. Thiolation in arsenic metabolism: a chemical perspective. Metallomics. 2018;10(10):1368–82.
Shen S, et al. Arsenic binding to proteins. Chem Rev. 2013;113(10):7769–92.
Drobna Z, et al. Disruption of the arsenic (+ 3 oxidation state) methyltransferase gene in the mouse alters the phenotype for methylation of arsenic and affects distribution and retention of orally administered arsenate. Chem Res Toxicol. 2009;22(10):1713–20.
Styblo M, et al. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch Toxicol. 2000;74(6):289–99.
Naranmandura H, Ibata K, Suzuki KT. Toxicity of dimethylmonothioarsinic acid toward human epidermoid carcinoma A431 cells. Chem Res Toxicol. 2007;20(8):1120–5.
Kim Y-T, et al. Kinetics of dimethylated thioarsenicals and the formation of highly toxic dimethylmonothioarsinic acid in environment. Environ Sci Technol. 2016;50(21):11637–45.
Rosen BP. Biochemistry of arsenic detoxification. FEBS Lett. 2002;529(1):86–92.
Willsky GR, Malamy MH. Effect of arsenate on inorganic phosphate transport in Escherichia coli. J Bacteriol. 1980;144(1):366–74.
Qin J, et al. Arsenic detoxification and evolution of trimethylarsine gas by a microbial arsenite S-adenosylmethionine methyltransferase. Proc Natl Acad Sci. 2006;103(7):2075–80.
Coryell M, Roggenbeck BA, Walk ST. The Human Gut Microbiome’s Influence on Arsenic Toxicity. Curr Pharmacol Rep. 2019;5(6):491–504 This review article provided a detalied discussion about Bacterial Arsenic Metabolism.
Neyt C, et al. Virulence and arsenic resistance in Yersiniae. J Bacteriol. 1997;179(3):612–9.
Chen J, Bhattacharjee H, Rosen BP. ArsH is an organoarsenical oxidase that confers resistance to trivalent forms of the herbicide monosodium methylarsenate and the poultry growth promoter roxarsone. Mol Microbiol. 2015;96(5):1042–52.
Chen J, et al. ArsP: a methylarsenite efflux permease. Mol Microbiol. 2015;98(4):625–35.
McDermott TR, Stolz JF, Oremland RS. Arsenic and the gastrointestinal tract microbiome. Environ Microbiol Rep. 2020;12(2):136–59 This review article discussed interactions between microbes and arsenic, particularly about genes of arsenic metabolism in bacteria.
Yoshinaga M, Rosen BP. AC· As lyase for degradation of environmental organoarsenical herbicides and animal husbandry growth promoters. Proc Natl Acad Sci. 2014;111(21):7701–6.
Lett M-C, et al. Unified nomenclature for genes involved in prokaryotic aerobic arsenite oxidation. J Bacteriol. 2012;194(2):207–8.
Liu G, et al. A periplasmic arsenite-binding protein involved in regulating arsenite oxidation. Environ Microbiol. 2012;14(7):1624–34.
Kashyap DR, et al. Complex regulation of arsenite oxidation in Agrobacterium tumefaciens. J Bacteriol. 2006;188(3):1081–8.
Koechler S, et al. Multiple controls affect arsenite oxidase gene expression in Herminiimonas arsenicoxydans. BMC Microbiol. 2010;10(1):53.
Zargar K, et al. Identification of a novel arsenite oxidase gene, arxA, in the haloalkaliphilic, arsenite-oxidizing bacterium Alkalilimnicola ehrlichii strain MLHE-1. J Bacteriol. 2010;192(14):3755–62.
Murphy JN, Durbin KJ, Saltikov CW. Functional Roles of arcA, etrA, Cyclic AMP (cAMP)-cAMP Receptor Protein, and cya in the Arsenate Respiration Pathway in Shewanella sp. Strain ANA-3. J Bacteriol. 2009;191(3):1035–43.
Murphy JN, Saltikov CW. The ArsR repressor mediates arsenite-dependent regulation of arsenate respiration and detoxification operons of Shewanella sp. strain ANA-3. J Bacteriol. 2009;191(21):6722–31.
Murphy JN, Saltikov CW. The ArsR repressor mediates arsenite-dependent regulation of arsenate respiration and detoxification operons of Shewanella sp. Strain ANA-3. J Bacteriol. 2009;191(21):6722–31.
DC. Rubin SS, et al. Arsenic thiolation and the role of sulfate-reducing bacteria from the human intestinal tract. Environ Health Perspect. 2014;122(8):817–22.
Lu K, et al. Gut microbiome perturbations induced by bacterial infection affect arsenic biotransformation. Chem Res Toxicol. 2013;26(12):1893–903.
Lu K, et al. Gut microbiome phenotypes driven by host genetics affect arsenic metabolism. Chem Res Toxicol. 2014;27(2):172–4.
Coryell M, et al. The gut microbiome is required for full protection against acute arsenic toxicity in mouse models. Nat Commun. 2018;9(1):5424.
Chi L, et al. Gut microbiome disruption altered the biotransformation and liver toxicity of arsenic in mice. Arch Toxicol. 2019;93(1):25–35 This article used conventional mice and gut microbiome-disrupted mice to explore the role of the gut microbiota in iAs biotransformation and its toxicity.
Zhou G-W, et al. Arsenic transformation mediated by gut microbiota affects the fecundity of Caenorhabditis elegans. Environ Pollut. 2020;260:113991.
Van de Wiele T, et al. Arsenic metabolism by human gut microbiota upon in vitro digestion of contaminated soils. Environ Health Perspect. 2010;118(7):1004–9.
Yu H, et al. Arsenic metabolism and toxicity influenced by ferric iron in simulated gastrointestinal tract and the roles of gut microbiota. Environ Sci Technol. 2016;50(13):7189–97.
Isokpehi RD, et al. Evaluative profiling of arsenic sensing and regulatory systems in the human microbiome project genomes. Microbiol Insights. 2014;7:MBI. S18076.
Chen Y, et al. Functional gene arrays-based analysis of fecal microbiomes in patients with liver cirrhosis. BMC Genomics. 2014;15(1):753.
Coryell M, et al. The gut microbiome is required for full protection against acute arsenic toxicity in mouse models. Nat Commun. 2018;9(1):1–9.
Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19(1):217–46.
Lin Y-C, et al. Association of plasma folate, vitamin B12 levels, and arsenic methylation capacity with developmental delay in preschool children in Taiwan. Arch Toxicol. 2019;93(9):2535–44.
Putnam EE, Goodman AL. B vitamin acquisition by gut commensal bacteria. PLoS Pathog. 2020;16(1):e1008208.
Gurwara S, et al. Dietary nutrients involved in one-carbon metabolism and colonic mucosa-associated gut microbiome in individuals with an endoscopically normal colon. Nutrients. 2019;11(3):613.
Howe CG, et al. Dietary B vitamin intake is associated with lower urinary monomethyl arsenic and oxidative stress marker 15-f2t-isoprostane among New Hampshire adults. J Nutr. 2017;147(12):2289–96.
Gamble MV, et al. Folate and arsenic metabolism: a double-blind, placebo-controlled folic acid–supplementation trial in Bangladesh. Am J Clin Nutr. 2006;84(5):1093–101.
Peters BA, et al. Folic acid and creatine as therapeutic approaches to lower blood arsenic: a randomized controlled trial. Environ Health Perspect. 2015;123(12):1294–301.
Mendez MA, et al. B-vitamins influence arsenic metabolism in Mexico. Hoboken: Wiley Online Library; 2013.
Wang Y, et al. Effects of exogenous glutathione on arsenic burden and NO metabolism in brain of mice exposed to arsenite through drinking water. Arch Toxicol. 2011;85(3):177–84.
Paul DS, et al. Molecular mechanisms of the diabetogenic effects of arsenic: inhibition of insulin signaling by arsenite and methylarsonous acid. Environ Health Perspect. 2007;115(5):734–42.
Huang M, Douillet C, Stýblo M. Arsenite and its trivalent methylated metabolites inhibit glucose-stimulated calcium influx and insulin secretion in murine pancreatic islets. Arch Toxicol. 2019;93(9):2525–33.
Dheer R, et al. Arsenic induces structural and compositional colonic microbiome change and promotes host nitrogen and amino acid metabolism. Toxicol Appl Pharmacol. 2015;289(3):397–408.
Seltenrich N. Arsenic and diabetes: assessing risk at low-to-moderate exposures. Environ Health Perspect. 2018;126(4):044002.
Tseng C-H. Arsenic methylation, urinary arsenic metabolites and human diseases: current perspective. J Environ Sci Health C. 2007;25(1):1–22.
Wei B, et al. Effects of arsenic methylation and metabolism on the changes of arsenic-related skin lesions. Environ Sci Pollut Res. 2018;25(24):24394–402.
Lu K, et al. Arsenic exposure perturbs the gut microbiome and its metabolic profile in mice: an integrated metagenomics and metabolomics analysis. Environ Health Perspect. 2014;122(3):284–91.
Martin AM, et al. The gut microbiome regulates host glucose homeostasis via peripheral serotonin. Proc Natl Acad Sci. 2019;116(40):19802–4.
Caricilli AM, Saad MJ. The role of gut microbiota on insulin resistance. Nutrients. 2013;5(3):829–51.
Bunderson M, Coffin JD, Beall HD. Arsenic induces peroxynitrite generation and cyclooxygenase-2 protein expression in aortic endothelial cells: possible role in atherosclerosis. Toxicol Appl Pharmacol. 2002;184(1):11–8.
Ding W, Hudson LG, Liu KJ. Inorganic arsenic compounds cause oxidative damage to DNA and protein by inducing ROS and RNS generation in human keratinocytes. Mol Cell Biochem. 2005;279(1-2):105–12.
Hu Y, et al. The role of reactive oxygen species in arsenic toxicity. Biomolecules. 2020;10(2):240.
Flora SJ, Mehta A, Gupta R. Prevention of arsenic-induced hepatic apoptosis by concomitant administration of garlic extracts in mice. Chem Biol Interact. 2009;177(3):227–33.
Chi L, et al. The effects of an environmentally relevant level of arsenic on the gut microbiome and its functional metagenome. Toxicol Sci. 2017;160(2):193–204.
Chattopadhyay S, et al. Apoptosis and necrosis in developing brain cells due to arsenic toxicity and protection with antioxidants. Toxicol Lett. 2002;136(1):65–76.
Simeonova PP, Luster MI. Mechanisms of arsenic carcinogenicity: genetic or epigenetic mechanisms? J Environ Pathol Toxicol Oncol. 2000;19(3):281–6.
Funding
This work was support in part by the NIH grant (R01ES024950), the UNC-Superfund Research Program funding (P42-ES-031007), and the University of North Carolina Center for Environmental Health and Susceptibility with the NIH grant (P30ES010126).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Metals and Health
Rights and permissions
About this article
Cite this article
Yang, Y., Chi, L., Lai, Y. et al. The gut microbiome and arsenic-induced disease—iAs metabolism in mice. Curr Envir Health Rpt 8, 89–97 (2021). https://doi.org/10.1007/s40572-021-00305-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40572-021-00305-9