Abstract
Purpose of Review
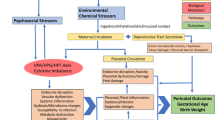
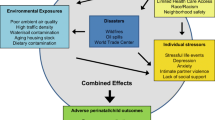
Pregnant women are exposed to numerous synthetic chemicals (e.g., pesticides, phthalates, polychlorinated biphenyls) in their daily lives as well as a range of non-chemical stressors, including poverty, depression, discrimination, and stressful life events. Although many studies have examined individual exposures to chemical and non-chemical stressors in relation to child health outcomes, very few studies have considered these exposures together. Here, we review the recent epidemiologic literature on the joint impact of chemical and non-chemical stressors on child outcomes.
Recent Findings
Considerable co-exposure to chemical and non-chemical stressors occurs in vulnerable populations. Non-chemical stressors may modify the impact of chemical exposures on children’s health, typically exacerbating their negative impact, but associations differ considerably by the chemicals and populations of interest.
Summary
Additional research is urgently needed to better understand the cumulative risks of multiple stressors on children’s health and the underlying physiological mechanisms.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Lichtveld K, Thomas K, Tulve NS. Chemical and non-chemical stressors affecting childhood obesity: a systematic scoping review. J Expo Sci Environ Epidemiol. 2018;28(1):1–12. Up to date review of factors related to childhood obesity with a focus on lifestyle and "obesogenic" environmental chemicals.
•• Burris HH, Hacker MR. Birth outcome racial disparities: a result of intersecting social and environmental factors. Semin Perinatol. 2017;41(6):360–6. Reviews well-documented disparities in birth outcomes in relation to environmental and psychosocial stressors, noting a need for research on interactions between the two.
Sexton K, Olden K, Johnson BL. “Environmental justice”: the central role of research in establishing a credible scientific foundation for informed decision making. Toxicol Ind Health. 1993;9(5):685–727.
• Morello-Frosch R, Shenassa ED. The environmental “riskscape” and social inequality: implications for explaining maternal and child health disparities. Environ Health Perspect. 2006;114(8):1150–3. Introduces a framework for thinking about the "double jeopardy" of co-exposure to environmental hazard and place-based stressors.
Ferguson KK, Chin HB. Environmental chemicals and preterm birth: biological mechanisms and the state of the science. Curr Epidemiol Rep. 2017;4(1):56–71.
Hobel CJ, Goldstein A, Barrett ES. Psychosocial stress and pregnancy outcome. Clin Obstet Gynecol. 2008;51(2):333–48.
Mitro SD, Johnson T, Zota AR. Cumulative chemical exposures during pregnancy and early development. Curr Environ Health Rep. 2015;2(4):367–78.
Entringer S, Buss C, Wadhwa PD. Prenatal stress, development, health and disease risk: a psychobiological perspective-2015 Curt Richter Award Paper. Psychoneuroendocrinology. 2015;62:366–75.
Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003-2004. Environ Health Perspect. 2011;119(6):878–85.
Haug LS, Sakhi AK, Cequier E, Casas M, Maitre L, Basagana X, et al. In-utero and childhood chemical exposome in six European mother-child cohorts. Environ Int. 2018;121(Pt 1):751–63.
Lilienfeld SO. Microaggressions. Perspect Psychol Sci. 2017;12(1):138–69.
Bowleg L. The problem with the phrase women and minorities: intersectionality-an important theoretical framework for public health. Am J Public Health. 2012;102(7):1267–73.
Shmool JL, Yonas MA, Newman OD, Kubzansky LD, Joseph E, Parks A, et al. Identifying perceived neighborhood stressors across diverse communities in New York City. Am J Community Psychol. 2015;56(1-2):145–55.
Seng JS, Lopez WD, Sperlich M, Hamama L, Reed Meldrum CD. Marginalized identities, discrimination burden, and mental health: empirical exploration of an interpersonal-level approach to modeling intersectionality. Soc Sci Med. 2012;75(12):2437–45.
Walsemann KM, Child S, Heck K, Margerison-Zilko C, Braveman P, Marchi K, et al. Are the poverty histories of neighbourhoods associated with psychosocial well-being among a representative sample of California mothers? An observational study. J Epidemiol Community Health. 2017;71(6):558–64.
Bhatia N, Chao SM, Higgins C, Patel S, Crespi CM. Association of mothers’ perception of neighborhood quality and maternal resilience with risk of preterm birth. Int J Environ Res Public Health. 2015;12(8):9427–43.
Geronimus AT. Understanding and eliminating racial inequalities in women’s health in the United States: the role of the weathering conceptual framework. J Am Med Women’s Assoc (1972). 2001;56(4):133–6 49-50.
Hogue CJ, Bremner JD. Stress model for research into preterm delivery among black women. Am J Obstet Gynecol. 2005;192(5 Suppl):S47–55.
Sealy-Jefferson S, Giurgescu C, Slaughter-Acey J, Caldwell C, Misra D. Neighborhood context and preterm delivery among African American women: the mediating role of psychosocial factors. J Urban Health. 2016;93(6):984–96.
Thayer ZM. Dark shadow of the long white cloud: neighborhood safety is associated with self-rated health and cortisol during pregnancy in Auckland, Aotearoa/New Zealand. SSM Popul Health. 2017;3:75–80.
•• Payne-Sturges DC, Scammell MK, Levy JI, Cory-Slechta DA, Symanski E, Carr Shmool JL, et al. Methods for evaluating the combined effects of chemical and nonchemical exposures for cumulative environmental health risk assessment. Int J Environ Res Public Health. 2018;15(12). Discussion of culmulative risk assessment as an approach to examining joint exposures to chemical and nonchemical stressors.
Ruiz D, Becerra M, Jagai JS, Ard K, Sargis RM. Disparities in environmental exposures to endocrine-disrupting chemicals and diabetes risk in vulnerable populations. Diabetes Care. 2018;41(1):193–205.
Attina TM, Malits J, Naidu M, Trasande L. Racial/ethnic disparities in disease burden and costs related to exposure to endocrine-disrupting chemicals in the United States: an exploratory analysis. J Clin Epidemiol. 2019;108:34–43.
Whitworth KW, Marshall AK, Symanski E. Maternal residential proximity to unconventional gas development and perinatal outcomes among a diverse urban population in Texas. PLoS One. 2017;12(7):e0180966.
Zota AR, Shamasunder B (2017) The environmental injustice of beauty: framing chemical exposures from beauty products as a health disparities concern. Am J Obstet Gynecol 217(4):418.e1–418.e6. https://doi.org/10.1016/j.ajog.2017.07.020
Nelson JW, Scammell MK, Hatch EE, Webster TF. Social disparities in exposures to bisphenol A and polyfluoroalkyl chemicals: a cross-sectional study within NHANES 2003-2006. Environ Health. 2012;11:10.
Zota AR, Phillips CA, Mitro SD. Recent fast food consumption and bisphenol A and phthalates exposures among the U.S. population in NHANES, 2003-2010. Environ Health Perspect. 2016;124(10):1521–8.
Bradman A, Quiros-Alcala L, Castorina R, Aguilar Schall R, Camacho J, Holland NT, et al. Effect of organic diet intervention on pesticide exposures in young children living in low-income urban and agricultural communities. Environ Health Perspect. 2015;123(10):1086–93.
Lu C, Barr DB, Pearson MA, Waller LA. Dietary intake and its contribution to longitudinal organophosphorus pesticide exposure in urban/suburban children. Environ Health Perspect. 2008;116(4):537–42.
Luo D, Pu Y, Tian H, Cheng J, Zhou T, Tao Y, et al. Concentrations of organochlorine pesticides in umbilical cord blood and related lifestyle and dietary intake factors among pregnant women of the Huaihe River Basin in China. Environ Int. 2016;92-93:276–83.
Padula AM, Monk C, Brennan PA, Borders A, Barrett ES, CT M, et al. A review of maternal prenatal exposures to environmental chemicals and psychosocial stressors - implications for research on perinatal outcomes in the ECHO Program. J Perinatol. 2019:In Press.
Zota AR, Geller RJ, Romano LE, Coleman-Phox K, Adler NE, Parry E, et al. Association between persistent endocrine-disrupting chemicals (PBDEs, OH-PBDEs, PCBs, and PFASs) and biomarkers of inflammation and cellular aging during pregnancy and postpartum. Environ Int. 2018;115:9–20.
Center for Disease Control and Prevention. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2019 [Available from: https://www.cdc.gov/exposurereport/].
Eick SM, Barrett ES, van ‘t Erve TJ, RHN N, Bush NR, Milne G, et al. Association between prenatal psychological stress and oxidative stress during pregnancy. Paediatr Perinat Epidemiol. 2018;32(4):318–26.
van T Erve TJ, Rosen EM, Barrett ES, Nguyen RHN, Sathyanarayana S, Milne GL, et al. Phthalates and phthalate alternatives have diverse associations with oxidative stress and inflammation in pregnant women. Environ Sci Technol. 2019;53(6):3258–67.
Chan JC, Nugent BM, Bale TL. Parental advisory: maternal and paternal stress can impact offspring neurodevelopment. Biol Psychiatry. 2018;83(10):886–94.
Rock KD, Patisaul HB. Environmental mechanisms of neurodevelopmental toxicity. Curr Environ Health Rep. 2018;5(1):145–57.
Cakmak S, Hebbern C, Cakmak JD, Vanos J. The modifying effect of socioeconomic status on the relationship between traffic, air pollution and respiratory health in elementary schoolchildren. J Environ Manag. 2016;177:1–8.
Erickson AC, Ostry A, Chan LH, Arbour L. The reduction of birth weight by fine particulate matter and its modification by maternal and neighbourhood-level factors: a multilevel analysis in British Columbia, Canada. Environ Health. 2016;15:51.
Nardone A, Neophytou AM, Balmes J, Thakur N. Ambient air pollution and asthma-related outcomes in children of color of the USA: a scoping review of literature published between 2013 and 2017. Curr Allergy Asthma Rep. 2018;18(5):29.
Rumchev K, Zhao Y, Spickett J. Health risk assessment of indoor air quality, socioeconomic and house characteristics on respiratory health among women and children of Tirupur, South India. Int J Environ Res Public Health. 2017;14(4).
Westergaard N, Gehring U, Slama R, Pedersen M. Ambient air pollution and low birth weight - are some women more vulnerable than others? Environ Int. 2017;104:146–54.
Rosa MJ, Just AC, Kloog I, Pantic I, Schnaas L, Lee A, et al. Prenatal particulate matter exposure and wheeze in Mexican children: effect modification by prenatal psychosocial stress. Ann Allergy Asthma Immunol. 2017;119(3):232–7 e1.
Brunst KJ, Sanchez-Guerra M, Chiu YM, Wilson A, Coull BA, Kloog I, et al. Prenatal particulate matter exposure and mitochondrial dysfunction at the maternal-fetal interface: effect modification by maternal lifetime trauma and child sex. Environ Int. 2018;112:49–58.
Padula AM, Mortimer KM, Tager IB, Hammond SK, Lurmann FW, Yang W, et al. Traffic-related air pollution and risk of preterm birth in the San Joaquin Valley of California. Ann Epidemiol. 2014;24(12):888–95e4.
Padula AM, Yang W, Carmichael SL, Tager IB, Lurmann F, Hammond SK, et al. Air pollution, neighbourhood socioeconomic factors, and neural tube defects in the San Joaquin Valley of California. Paediatr Perinat Epidemiol. 2015;29(6):536–45.
O’ Lenick CR, Chang HH, Kramer MR, Winquist A, Mulholland JA, Friberg MD, et al. Ozone and childhood respiratory disease in three US cities: evaluation of effect measure modification by neighborhood socioeconomic status using a Bayesian hierarchical approach. Environ Health. 2017;16(1):36.
Deguen S, Ahlers N, Gilles M, Danzon A, Carayol M, Zmirou-Navier D, et al. Using a clustering approach to investigate socio-environmental inequality in preterm birth-a study conducted at fine spatial scale in Paris (France). Int J Environ Res Public Health. 2018;15(9).
Morelli X, Rieux C, Cyrys J, Forsberg B, Slama R. Air pollution, health and social deprivation: a fine-scale risk assessment. Environ Res. 2016;147:59–70.
Oudin A, Braback L, Oudin Astrom D, Forsberg B. Air pollution and dispensed medications for asthma, and possible effect modifiers related to mental health and socio-economy: a longitudinal cohort study of Swedish children and adolescents. Int J Environ Res Public Health. 2017;14(11).
Padilla CM, Kihal-Talantikit W, Vieira VM, Deguen S. City-specific spatiotemporal infant and neonatal mortality clusters: links with socioeconomic and air pollution spatial patterns in France. Int J Environ Res Public Health. 2016;13(6).
Pratt GC, Vadali ML, Kvale DL, Ellickson KM. Traffic, air pollution, minority and socio-economic status: addressing inequities in exposure and risk. Int J Environ Res Public Health. 2015;12(5):5355–72.
Shmool JL, Kubzansky LD, Newman OD, Spengler J, Shepard P, Clougherty JE. Social stressors and air pollution across New York City communities: a spatial approach for assessing correlations among multiple exposures. Environ Health. 2014;13:91.
Stieb DM, Chen L, Hystad P, Beckerman BS, Jerrett M, Tjepkema M, et al. A national study of the association between traffic-related air pollution and adverse pregnancy outcomes in Canada, 1999-2008. Environ Res. 2016;148:513–26.
Choe SA, Kauderer S, Eliot MN, Glazer KB, Kingsley SL, Carlson L, et al. Air pollution, land use, and complications of pregnancy. Sci Total Environ. 2018;645:1057–64.
Li L, Laurent O, Wu J. Spatial variability of the effect of air pollution on term birth weight: evaluating influential factors using Bayesian hierarchical models. Environ Health. 2016;15:14.
Costa LG, Giordano G, Guizzetti M, Vitalone A. Neurotoxicity of pesticides: a brief review. Front Biosci. 2008;13:1240–9.
Beijers R, Buitelaar JK, de Weerth C. Mechanisms underlying the effects of prenatal psychosocial stress on child outcomes: beyond the HPA axis. Eur Child Adolesc Psychiatry. 2014;23(10):943–56.
• Stein LJ, Gunier RB, Harley K, Kogut K, Bradman A, Eskenazi B. Early childhood adversity potentiates the adverse association between prenatal organophosphate pesticide exposure and child IQ: the CHAMACOS cohort. Neurotoxicology. 2016;56:180–7. Highlights sex-specific associations between prenatal pesticide exposure and non-chemical stressors on child IQ in the CHAMACOS cohort.
Rowe C, Gunier R, Bradman A, Harley KG, Kogut K, Parra K, et al. Residential proximity to organophosphate and carbamate pesticide use during pregnancy, poverty during childhood, and cognitive functioning in 10-year-old children. Environ Res. 2016;150:128–37.
Donauer S, Altaye M, Xu Y, Sucharew H, Succop P, Calafat AM, et al. An observational study to evaluate associations between low-level gestational exposure to organophosphate pesticides and cognition during early childhood. Am J Epidemiol. 2016;184(5):410–8.
Huang J, Eskenazi B, Bornman R, Rauch S, Chevrier J. Maternal peripartum serum DDT/E and urinary pyrethroid metabolite concentrations and child infections at 2 years in the VHEMBE birth cohort. Environ Health Perspect. 2018;126(6):067006.
Albouy-Llaty M, Limousi F, Carles C, Dupuis A, Rabouan S, Migeot V. Association between exposure to endocrine disruptors in drinking water and preterm birth, taking neighborhood deprivation into account: a historic cohort study. Int J Environ Res Public Health. 2016;13(8).
Korfmacher J, O’Brien R, Hiatt S, Olds D. Differences in program implementation between nurses and paraprofessionals providing home visits during pregnancy and infancy: a randomized trial. Am J Public Health. 1999;89(12):1847–51.
Valvi D, Monfort N, Ventura R, Casas M, Casas L, Sunyer J, et al. Variability and predictors of urinary phthalate metabolites in Spanish pregnant women. Int J Hyg Environ Health. 2015;218(2):220–31.
•• Zota AR, Shamasunder B. The environmental injustice of beauty: framing chemical exposures from beauty products as a health disparities concern. Am J Obstet Gynecol. 2017;217(4):418 e1–6. Examines sociodemographic disparities in chemical exposures through personal care products.
Erkekoglu P, Kocer-Gumusel B. Genotoxicity of phthalates. Toxicol Mech Methods. 2014;24(9):616–26.
Hansen JF, Bendtzen K, Boas M, Frederiksen H, Nielsen CH, Rasmussen AK, et al. Influence of phthalates on cytokine production in monocytes and macrophages: a systematic review of experimental trials. PLoS One. 2015;10(3):e0120083.
Zarean M, Keikha M, Feizi A, Kazemitabaee M, Kelishadi R. The role of exposure to phthalates in variations of anogenital distance: a systematic review and meta-analysis. Environ Pollut. 2019;247:172–9.
Thankamony A1,2, Pasterski V1,3, Ong KK4, Acerini CL1, Hughes IA1 (2016) Anogenital distance as a marker of androgen exposure in humans. Andrology 4(4):616-25. https://doi.org/10.1111/andr.12156
Drake AJ, van den Driesche S, Scott HM, Hutchison GR, Seckl JR, Sharpe RM. Glucocorticoids amplify dibutyl phthalate-induced disruption of testosterone production and male reproductive development. Endocrinology. 2009;150(11):5055–64.
• Barrett ES, Parlett LE, Sathyanarayana S, Redmon JB, Nguyen RH, Swan SH. Prenatal stress as a modifier of associations between phthalate exposure and reproductive development: results from a multicentre pregnancy cohort study. Paediatr Perinat Epidemiol. 2016;30(2):105–14. Provides evidence suggesting that psychosocial stress may protect against the adverse impacts of phthalates on reproductive development.
Barrett ES, Swan SH. Stress and androgen activity during fetal development. Endocrinology. 2015;156(10):3435–41.
Wenzel AG, Bloom MS, Butts CD, Wineland RJ, Brock JW, Cruze L, et al. Influence of race on prenatal phthalate exposure and anogenital measurements among boys and girls. Environ Int. 2018;110:61–70.
James-Todd TM, Meeker JD, Huang T, Hauser R, Seely EW, Ferguson KK, et al. Racial and ethnic variations in phthalate metabolite concentration changes across full-term pregnancies. J Expo Sci Environ Epidemiol. 2017;27(2):160–6.
Bloom MS, Wenzel AG, Brock JW, Kucklick JR, Wineland RJ, Cruze L, et al. Racial disparity in maternal phthalates exposure; association with racial disparity in fetal growth and birth outcomes. Environ Int. 2019;127:473–86.
Patel JF, Hartman TJ, Sjodin A, Northstone K, Taylor EV. Prenatal exposure to polychlorinated biphenyls and fetal growth in British girls. Environ Int. 2018;116:116–21.
•• Padula AM, Huang H, Baer RJ, August LM, Jankowska MM, Jellife-Pawlowski LL, et al. Environmental pollution and social factors as contributors to preterm birth in Fresno County. Environ Health. 2018;17(1):70. A large study of California births suggesting that the relationship between environmental pollution and preterm birth is the strongest in low SES areas.
Rappazzo KM, Messer LC, Jagai JS, Gray CL, Grabich SC, Lobdell DT. The associations between environmental quality and preterm birth in the United States, 2000-2005: a cross-sectional analysis. Environ Health. 2015;14:50.
Office of Environmental Hazard and Health Assessment. CalEnviroScreen [Available from: https://oehha.ca.gov/calenviroscreen].
Juarez PD, Matthews-Juarez P, Hood DB, Im W, Levine RS, Kilbourne BJ, et al. The public health exposome: a population-based, exposure science approach to health disparities research. Int J Environ Res Public Health. 2014;11(12):12866–95.
Huang H, Wang A, Morello-Frosch R, Lam J, Sirota M, Padula A, et al. Cumulative risk and impact modeling on environmental chemical and social stressors. Curr Environ Health Rep. 2018;5(1):88–99.
•• Cowell WJ, Wright RJ. Sex-specific effects of combined exposure to chemical and non-chemical stressors on neuroendocrine development: a review of recent findings and putative mechanisms. Curr Environ Health Rep. 2017;4(4):415–25. A review of the animal and human literature on combined exposure to chemical and non-chemical stressors a focus on neurodevelopmental outcomes.
Funding
Support for this work was provided by NIH P30ES005022, R00ES021470.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Synthetic Chemicals and Health
Rights and permissions
About this article
Cite this article
Barrett, E.S., Padula, A.M. Joint Impact of Synthetic Chemical and Non-chemical Stressors on Children’s Health. Curr Envir Health Rpt 6, 225–235 (2019). https://doi.org/10.1007/s40572-019-00252-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40572-019-00252-6