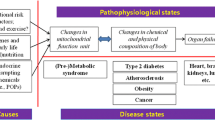
Abstract
Purpose of Review
Environmental toxicants are increasingly implicated in the global decline in metabolic health. Focusing on diabetes, herein, the molecular and cellular mechanisms by which metabolism disrupting chemicals (MDCs) impair energy homeostasis are discussed.
Recent Findings
Emerging data implicate MDC perturbations in a variety of pathways as contributors to metabolic disease pathogenesis, with effects in diverse tissues regulating fuel utilization. Potentiation of traditional metabolic risk factors, such as caloric excess, and emerging threats to metabolism, such as disruptions in circadian rhythms, are important areas of current and future MDC research. Increasing evidence also implicates deleterious effects of MDCs on metabolic programming that occur during vulnerable developmental windows, such as in utero and early post-natal life as well as pregnancy.
Summary
Recent insights into the mechanisms by which MDCs alter energy homeostasis will advance the field’s ability to predict interactions with classical metabolic disease risk factors and empower studies utilizing targeted therapeutics to treat MDC-mediated diabetes.

Similar content being viewed by others
Abbreviations
- EDCs:
-
Endocrine disrupting chemicals
- GDM:
-
Gestational diabetes mellitus
- MDCs:
-
Metabolism disrupting chemicals
- T1DM:
-
Type 1 diabetes mellitus
- T2DM:
-
Type 2 diabetes mellitus
- GLUT2:
-
glucose transporter 2
- GI:
-
Gastrointestinal
- ATP:
-
Adenosine triphosphate
- ADP:
-
Adenosine diphosphate
- KATP :
-
Potassium sensitive ATP channel
- TPT:
-
Triphenyltin
- GLP-1:
-
Glucagon-like peptide-1
- GIP:
-
Gastric inhibitory polypeptide/glucose-dependent insulinotropic polypeptide
- cAMP:
-
Cyclic AMP
- NAD(P)H:
-
Nicotinamide adenine dinucleotide phosphate
- SNAP-25:
-
Synaptosome-associated protein 25 kDa
- GPCR:
-
G-Protein-coupled receptor
- TBT:
-
Tributyltin
- PKA:
-
Protein kinase A
- PCB:
-
Polychlorinated biphenyl
- CamKII:
-
Calcium/calmodulin-dependent protein kinase II
- cGMP:
-
cyclic guanosine monophosphate
- BPA:
-
Bisphenol A
- GSIS:
-
Glucose-stimulated insulin secretion
- PAH:
-
Polycyclic aromatic hydrocarbon
- ncmER:
-
non-classical membrane estrogen receptor
- ERβ:
-
Estrogen receptor beta
- CREB:
-
response element binding protein
- ERα:
-
Estrogen receptor alpha
- ERK:
-
Extracellular signal-regulated kinase
- ER:
-
Endoplasmic reticulum
- DEHP:
-
Diethylhexylphthalate
- ROS:
-
Reactive oxygen species
- DNA:
-
Deoxyribonucleic acid
- NAC:
-
N-Acetylcysteine
- JNK:
-
c-Jun N-terminal kinase
- NOD:
-
Non-obese diabetic
- IRS:
-
Insulin receptor substrate
- PI3-K:
-
Phosphatidylinositol 3 kinase
- PIP3 :
-
Phosphatidylinositol triphosphate
- PDK1:
-
Phosphoinositide-dependent kinase 1
- PKB:
-
Protein Kinase B
- PM:
-
Particulate matter
- TCDD:
-
2,3,7,8-Tetrachlorodibenzo dioxin
- TF:
-
Tolylfluanid
- POP:
-
Persistent organic pollutant
- PFOA:
-
Perfluorooctanoic acid
- PEPCK:
-
Phosphoenolpyruvate carboxykinase
- AhR:
-
Aryl hydrocarbon receptor
- PFOS:
-
Perfluorooctanesulfonic acid
- HNF4α:
-
Hepatocyte nuclear factor 4 alpha
- PPARγ:
-
Peroxisome proliferator activated receptor gamma
- GR:
-
glucocorticoid receptor
- TNFα:
-
Tumor necrosis factor alpha
- AMPK:
-
5′ Adenosine monophosphate-activated protein kinase
- IL-1β:
-
Interleukin 1 beta
- NFκB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- MAPK:
-
Mitogen-activated protein kinase
- IL-6:
-
Interleukin 6
- NEFA:
-
Non-esterified fatty acids
- Srebpc1:
-
Sterol regulatory element-binding protein 1
- PPARα:
-
Peroxisome proliferator activated receptor alpha
- Cpt1b:
-
Carnitine palmitoyltransferase 1B
- CDK4:
-
Cyclin-dependent kinase-4
- SGLT-2:
-
Sodium-glucose cotransporter-2
- DPP4:
-
Dipeptidyl peptidase 4
- GLP-1:
-
Glucagon-like peptide-1
References
Papers of Particular Interest, Published Recently, Have Been Highlighted as: • Of Importance •• Of Outstanding Importance
Dabelea D, Mayer-Davis EJ, Saydah S, Imperatore G, Linder B, Divers J, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311(17):1778–86.
DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, pregnancy risk assessment monitoring system (PRAMS), 2007-2010. Prev Chronic Dis. 2014;11:E104.
Damm P, Houshmand-Oeregaard A, Kelstrup L, Lauenborg J, Mathiesen ER, Clausen TD. Gestational diabetes mellitus and long-term consequences for mother and offspring: a view from Denmark. Diabetologia. 2016;59(7):1396–9.
•• Brown RE, Sharma AM, Ardern CI, Mirdamadi P, Mirdamadi P, Kuk JL. Secular differences in the association between caloric intake, macronutrient intake, and physical activity with obesity. Obesity Research & Clinical Practice. 2016;10(3):243–55. This study provides evidence that increasing rates of obesity are not accounted for by changes in diet or activity, supporting the need to pursue alternate etiologies.
•• Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. EDC-2: the endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015;36(6):E1–E150. Comprehensive review of endocrine disrupting chemicals across all organ systems.
Miura Y, Kato M, Ogino K, Matsui H. Impaired cytosolic Ca2+ response to glucose and gastric inhibitory polypeptide in pancreatic beta-cells from triphenyltin-induced diabetic hamster. Endocrinology. 1997;138(7):2769–75.
Miura Y, Hori Y, Kimura S, Hachiya H, Sakurai Y, Inoue K, et al. Triphenyltin impairs insulin secretion by decreasing glucose-induced NADP(H) and ATP production in hamster pancreatic β-cells. Toxicology. 2012;299(2–3):165–71.
Miura Y, Matsui H. Triphenyltin impairs a protein kinase A (PKA)-dependent increase of cytosolic Na+ and Ca2+ and PKA-independent increase of cytosolic Ca2+ associated with insulin secretion in hamster pancreatic beta-cells. Toxicol Appl Pharmacol. 2006;216(3):363–72.
Zuo Z, Wu T, Lin M, Zhang S, Yan F, Yang Z, et al. Chronic exposure to tributyltin chloride induces pancreatic islet cell apoptosis and disrupts glucose homeostasis in male mice. Environ Sci Technol. 2014;48(9):5179–86.
Zuo Z, Chen S, Wu T, Zhang J, Su Y, Chen Y, et al. Tributyltin causes obesity and hepatic steatosis in male mice. Environ Toxicol. 2011;26(1):79–85.
Regnier SM, El-Hashani E, Kamau W, Zhang X, Massad NL, Sargis RM. Tributyltin differentially promotes development of a phenotypically distinct adipocyte. Obesity. 2015;23(9):1864–71.
Grün F, Watanabe H, Zamanian Z, Maeda L, Arima K, Cubacha R, et al. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol Endocrinol. 2006;20(9):2141–55.
Kirchner S, Kieu T, Chow C, Casey S, Blumberg B. Prenatal exposure to the environmental obesogen tributyltin predisposes multipotent stem cells to become adipocytes. Mol Endocrinol. 2010;24(3):526–39.
Douillet C, Currier J, Saunders J, Bodnar WM, Matoušek T, Stýblo M. Methylated trivalent arsenicals are potent inhibitors of glucose stimulated insulin secretion by murine pancreatic islets. Toxicol Appl Pharmacol. 2013;267(1):11–5.
Fu J, Woods CG, Yehuda-Shnaidman E, Zhang Q, Wong V, Collins S, et al. Low-level arsenic impairs glucose-stimulated insulin secretion in pancreatic beta cells: involvement of cellular adaptive response to oxidative stress. Environ Health Perspect. 2010;118(6):864–70.
Díaz-Villaseñor A, Burns AL, Salazar AM, Sordo M, Hiriart M, Cebrián ME, et al. Arsenite reduces insulin secretion in rat pancreatic beta-cells by decreasing the calcium-dependent calpain-10 proteolysis of SNAP-25. Toxicol Appl Pharmacol. 2008;231(3):291–9.
Díaz-Villaseñor A, Sánchez-Soto MC, Cebrián ME, Ostrosky-Wegman P, Hiriart M. Sodium arsenite impairs insulin secretion and transcription in pancreatic beta-cells. Toxicol Appl Pharmacol. 2006;214(1):30–4.
Zhu X-X, Yao X-F, Jiang L-P, Geng C-Y, Zhong L-F, Yang G, et al. Sodium arsenite induces ROS-dependent autophagic cell death in pancreatic β-cells. Food Chem Toxicol. 2014;70:144–50.
Yao X-F, Zheng B-L, Bai J, Jiang L-P, Zheng Y, Qi B-X, et al. Low-level sodium arsenite induces apoptosis through inhibiting TrxR activity in pancreatic β-cells. Environ Toxicol Pharmacol. 2015;40(2):486–91.
Paul DS, Harmon AW, Devesa V, Thomas DJ, Stýblo M. Molecular mechanisms of the diabetogenic effects of arsenic: inhibition of insulin signaling by arsenite and methylarsonous acid. Environ Health Perspect. 2007;115(5):734–42.
Xue P, Hou Y, Zhang Q, Woods CG, Yarborough K, Liu H, et al. Prolonged inorganic arsenite exposure suppresses insulin-stimulated AKT S473 phosphorylation and glucose uptake in 3T3-L1 adipocytes: involvement of the adaptive antioxidant response. Biochem Biophys Res Commun. 2011;407(2):360–5.
Huang C-F, Yang C-Y, Chan D-C, Wang C-C, Huang K-H, Wu C-C, et al. Arsenic exposure and glucose intolerance/insulin resistance in estrogen-deficient female mice. Environ Health Perspect. 2015;123(11):1138–44.
Rodriguez KF, Ungewitter EK, Crespo-Mejias Y, Liu C, Nicol B, Kissling GE, et al. Effects of in utero exposure to arsenic during the second half of gestation on reproductive end points and metabolic parameters in female CD-1 mice. Environ Health Perspect. 2016;124(3):336–43.
Dávila-Esqueda ME, Morales JMV, Jiménez-Capdeville ME, la Cruz DE, Falcón-Escobedo R, Chi-Ahumada E, et al. Low-level subchronic arsenic exposure from prenatal developmental stages to adult life results in an impaired glucose homeostasis. Exp Clin Endocrinol Diabetes. 2011;119(10):613–617–617.
Muayed El M, Raja MR, Zhang X, MacRenaris KW, Bhatt S, Chen X, et al. Accumulation of cadmium in insulin-producing β cells. Islets. 2012;4(6):405–16.
Chang K-C, Hsu C-C, Liu S-H, Su C-C, Yen C-C, Lee M-J, et al. Cadmium induces apoptosis in pancreatic β-cells through a mitochondria-dependent pathway: the role of oxidative stress-mediated c-Jun N-terminal kinase activation. PLoS One. 2013;8(2):e54374.
Han JC, Park SY, Hah BG, Choi GH, Kim YK, Kwon TH, et al. Cadmium induces impaired glucose tolerance in rat by down-regulating GLUT4 expression in adipocytes. Arch Biochem Biophys. 2003;413(2):213–20.
Treviño S, Waalkes MP, Flores Hernández JA, León-Chavez BA, Aguilar-Alonso P, Brambila E. Chronic cadmium exposure in rats produces pancreatic impairment and insulin resistance in multiple peripheral tissues. Arch Biochem Biophys. 2015;583:27–35.
Chen Y-W, Huang C-F, Yang C-Y, Yen C-C, Tsai K-S, Liu S-H. Inorganic mercury causes pancreatic beta-cell death via the oxidative stress-induced apoptotic and necrotic pathways. Toxicol Appl Pharmacol. 2010;243(3):323–31.
Chen Y-W, Huang C-F, Tsai K-S, Yang R-S, Yen C-C, Yang C-Y, et al. The role of phosphoinositide 3-kinase/Akt signaling in low-dose mercury-induced mouse pancreatic beta-cell dysfunction in vitro and in vivo. Diabetes. 2006;55(6):1614–24.
Song L, Xia W, Zhou Z, Li Y, Lin Y, Wei J, et al. Low-level phenolic estrogen pollutants impair islet morphology and β-cell function in isolated rat islets. J Endocrinol. 2012;215(2):303–11.
Kim J, Kang E-J, Park M-N, Kim J-E, Kim S-C, Jeung E-B, et al. The adverse effect of 4-tert-octylphenol on fat metabolism in pregnant rats via regulation of lipogenic proteins. Environ Toxicol Pharmacol. 2015;40(1):284–91.
Xin F, Jiang L, Liu X, Geng C, Wang W, Zhong L, et al. Bisphenol A induces oxidative stress-associated DNA damage in INS-1 cells. Mutat Res Genet Toxicol Environ Mutagen. 2014;769:29–33.
• Bodin J, Bolling AK, Becher R, Kuper F, Lovik M, Nygaard UC. Transmaternal bisphenol A exposure accelerates diabetes type 1 development in NOD mice. Toxicol Sci. 2014;137(2):311–23. This study demonstrates that in utero BPA exposure can exacerbate β-cell decline in a mouse model of Type 1 diabetes.
Batista TM, Alonso-Magdalena P, Vieira E, Amaral MEC, Cederroth CR, Nef S, et al. Short-term treatment with bisphenol-A leads to metabolic abnormalities in adult male mice. PLoS One. 2012;7(3):e33814.
Valentino R, D’Esposito V, Passaretti F, Liotti A, Cabaro S, Longo M, et al. Bisphenol-A impairs insulin action and up-regulates inflammatory pathways in human subcutaneous adipocytes and 3T3-L1 cells. PLoS ONE. Public Library of Science. 2013;8(12):e82099.
Moon MK, Jeong I-K, Jung Oh T, Ahn HY, Kim HH, Park YJ, et al. Long-term oral exposure to bisphenol A induces glucose intolerance and insulin resistance. J Endocrinol. 2015;226(1):35–42.
Indumathi D, Jayashree S, Selvaraj J, Sathish S, Mayilvanan C, Akilavalli N, et al. Effect of bisphenol-A on insulin signal transduction and glucose oxidation in skeletal muscle of adult male albino rat. Human & Experimental Toxicology. 2013;32(9):960–71.
Sakurai K, Kawazuma M, Adachi T, Harigaya T, Saito Y, Hashimoto N, et al. Bisphenol A affects glucose transport in mouse 3T3-F442A adipocytes. British Journal of Pharmacology Blackwell Publishing Ltd. 2004;141(2):209–14.
Ohlstein JF, Strong AL, McLachlan JA, Gimble JM, Burow ME, Bunnell BA. Bisphenol A enhances adipogenic differentiation of human adipose stromal/stem cells. J Mol Endocrinol. 2014;53(3):345–53.
• Ariemma F, D’Esposito V, Liguoro D, Oriente F, Cabaro S, Liotti A, et al. Low-dose bisphenol-A impairs adipogenesis and generates dysfunctional 3T3-L1 adipocytes. PLoS One. 2016;11(3):e0150762. This study demonstrates that BPA exposure can promote the genesis of dysfunctional adipocytes which are detrimental to metabolic health, an understudied area of MDC effects.
Alonso-Magdalena P, Vieira E, Soriano S, Menes L, Burks D, Quesada I, et al. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ Health Perspect. 2010;118(9):1243–50.
•• García-Arévalo M, Alonso-Magdalena P, Servitja J-M, Boronat-Belda T, Merino B, Villar-Pazos S, et al. Maternal exposure to bisphenol-A during pregnancy increases pancreatic β-cell growth during early life in male mice offspring. Endocrinology. 2016;157(11):4158–71. This study provides evidence that in utero BPA exposure leads to impaired glucose tolerance later in life, which is exacerbated by high fat feeding, demonstrating the interaction between traditional metabolic risk factors and gestational MDC exposure.
•• Alonso-Magdalena P, García-Arévalo M, Quesada I, Nadal A. Bisphenol-A treatment during pregnancy in mice: a new window of susceptibility for the development of diabetes in mothers later in life. Endocrinology. 2015;156(5):1659–70. This study demonstrates BPA exposure during pregnancy promotes diabetes development later in life and demonstrates that in addition to being a vulnerable window for the fetus, pregnancy is also a vulnerable window for mothers.
Sun X, Lin Y, Huang Q, Shi J, Qiu L, Kang M, et al. Di(2-ethylhexyl) phthalate-induced apoptosis in rat INS-1 cells is dependent on activation of endoplasmic reticulum stress and suppression of antioxidant protection. J Cell Mol Med. 2015;19(3):581–94.
Rajesh P, Balasubramanian K. Di(2-ethylhexyl)phthalate exposure impairs insulin receptor and glucose transporter 4 gene expression in L6 myotubes. Human & Experimental Toxicology. 2014;33(7):685–700.
Rajesh P, Sathish S, Srinivasan C, Selvaraj J, Balasubramanian K. Phthalate is associated with insulin resistance in adipose tissue of male rat: role of antioxidant vitamins. J Cell Biochem. 2013;114(3):558–69.
Martinelli MI, Mocchiutti NO, Bernal CA. Dietary di(2-ethylhexyl)phthalate-impaired glucose metabolism in experimental animals. Human & Experimental Toxicology. 2006;25(9):531–8.
Campioli E, Martinez-Arguelles DB, Papadopoulos V. In utero exposure to the endocrine disruptor di-(2-ethylhexyl) phthalate promotes local adipose and systemic inflammation in adult male offspring. Nutr Diabetes. 2014;4:e115.
Fischer LJ, Wagner MA, Madhukar BV. Potential involvement of calcium, CaM kinase II, and MAP kinases in PCB-stimulated insulin release from RINm5F cells. Toxicol Appl Pharmacol. 1999;159(3):194–203.
Baker NA, Karounos M, English V, Fang J, Wei Y, Stromberg A, et al. Coplanar polychlorinated biphenyls impair glucose homeostasis in lean C57BL/6 mice and mitigate beneficial effects of weight loss on glucose homeostasis in obese mice. Environ Health Perspect. 2013;121(1):105–10.
Gray SL, Shaw AC, Gagne AX, Chan HM. Chronic exposure to PCBs (Aroclor 1254) exacerbates obesity-induced insulin resistance and hyperinsulinemia in mice. J Toxicol Environ Health Part A. 2013;76(12):701–15.
Nishiumi S, Yoshida M, Azuma T, Yoshida KI, Ashida H. 2,3,7,8-Tetrachlorodibenzo-p-dioxin impairs an insulin signaling pathway through the induction of tumor necrosis factor-α in adipocytes. Toxicol Sci. 2010;115(2):482–91.
Liu PC, Matsumura F. Differential effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the “adipose- type” and “brain-type” glucose transporters in mice. Mol Pharmacol American Society for Pharmacology and Experimental Therapeutics. 1995;47(1):65–73.
Zhang W, Sargis RM, Volden PA, Carmean CM, Sun XJ, Brady MJ. PCB 126 and other dioxin-like PCBs specifically suppress hepatic PEPCK expression via the aryl hydrocarbon receptor. Andrabi SA, editor. PLoS One. 2012;7(5):e37103.
Novelli M, Piaggi S, De Tata V. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-induced impairment of glucose-stimulated insulin secretion in isolated rat pancreatic islets. Toxicol Lett. 2005;156(2):307–14.
Piaggi S, Novelli M, Martino L, Masini M, Raggi C, Orciuolo E, et al. Cell death and impairment of glucose-stimulated insulin secretion induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in the β-cell line INS-1E. Toxicol Appl Pharmacol. 2007;220(3):333–40.
Kim Y-H, Shim Y-J, Shin Y-J, Sul D, Lee E, Min B-H. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induces calcium influx through T-type calcium channel and enhances lysosomal exocytosis and insulin secretion in INS-1 cells. Int J Toxicol. 2009;28(3):151–61.
Kurita H, Yoshioka W, Nishimura N, Kubota N, Kadowaki T, Tohyama C. Aryl hydrocarbon receptor-mediated effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on glucose-stimulated insulin secretion in mice. Journal of Applied Toxicology John Wiley & Sons, Ltd. 2009;29(8):689–94.
Beggs KM, McGreal SR, McCarthy A, Gunewardena S, Lampe JN, Lau C, et al. The role of hepatocyte nuclear factor 4-alpha in perfluorooctanoic acid- and perfluorooctanesulfonic acid-induced hepatocellular dysfunction. Toxicol Appl Pharmacol. 2016;304:18–29.
Hines EP, White SS, Stanko JP, Gibbs-Flournoy EA, Lau C, Fenton SE. Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD-1 mice: low doses induce elevated serum leptin and insulin, and overweight in mid-life. Mol Cell Endocrinol. 2009;304(1–2):97–105.
Wan HT, Zhao YG, Leung PY, Wong CKC. Perinatal exposure to perfluorooctane sulfonate affects glucose metabolism in adult offspring. PLoS One. 2014;9(1):e87137.
Yan S, Zhang H, Zheng F, Sheng N, Guo X, Dai J. Perfluorooctanoic acid exposure for 28 days affects glucose homeostasis and induces insulin hypersensitivity in mice. Sci Rep. 2015.
Sargis RM, Neel BA, Brock CO, Lin Y, Hickey AT, Carlton DA, et al. The novel endocrine disruptor tolylfluanid impairs insulin signaling in primary rodent and human adipocytes through a reduction in insulin receptor substrate-1 levels. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2012;1822(6):952–60.
Sargis RM, Johnson DN, Choudhury RA, Brady MJ. Environmental endocrine disruptors promote adipogenesis in the 3T3-L1 cell line through glucocorticoid receptor activation. Obesity. 2010;18(7):1283–8.
• Regnier SM, Kirkley AG, Ye H, El-Hashani E, Zhang X, Neel BA, et al. Dietary exposure to the endocrine disruptor tolylfluanid promotes global metabolic dysfunction in male mice. Endocrinology. 2015;156(3):896–910. This study investigates the role of TF in disrupting glucocorticoid signaling, an understudied area of MDC disruption.
Jin Y, Lin X, Miao W, Wang L, Wu Y, Fu Z. Oral exposure of pubertal male mice to endocrine-disrupting chemicals alters fat metabolism in adult livers. Environ Toxicol. 2015;30(12):1434–44.
Lim S, Ahn SY, Song IC, Chung MH, Jang HC, Park KS, et al. Chronic exposure to the herbicide, atrazine, causes mitochondrial dysfunction and insulin resistance. PLoS One. 2009;4(4):e5186.
• Zheng Z, Xu X, Zhang X, Wang A, Zhang C, Hüttemann M, et al. Exposure to ambient particulate matter induces a NASH-like phenotype and impairs hepatic glucose metabolism in an animal model. J Hepatol. 2013;58(1):148–54. This study showed that air pollution can trigger hepatic steatosis and impair glucose metabolism, providing evidence for population studies linking metabolic disease to air quality.
Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, et al. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009;119(4):538–46.
Yan Y-H, Chou CC, Lee C-T, Liu J-Y, Cheng T-J. Enhanced insulin resistance in diet-induced obese rats exposed to fine particles by instillation. Inhal Toxicol. 2011;23(9):507–19.
da Rocha FJ, Ogurtsova K, Linnenkamp U, Guariguata L, Seuring T, Zhang P, et al. IDF Diabetes Atlas estimates of 2014 global health expenditures on diabetes. Diabetes Res Clin Pract. 2016;117:48–54.
Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature Nature Publishing Group. 2006;444(7121):840–6.
Fu Z, Gilbert ER, Liu D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr Diabetes Rev. 2013;9(1):25–53.
Schwartz SS, Epstein S, Corkey BE, Grant SFA, Gavin JR, Aguilar RB. The time is right for a new classification system for diabetes: rationale and implications of the β-cell-centric classification schema. Dia Care. 2016;39(2):179–86.
Yabe D, Seino Y. Two incretin hormones GLP-1 and GIP: comparison of their actions in insulin secretion and β cell preservation. Prog Biophys Mol Biol. 2011;107(2):248–56.
Shankar S, Shanker U, Shikha. Arsenic contamination of groundwater: a review of sources, prevalence, health risks, and strategies for mitigation. Scientific World Journal. 2014;2014:304524.
Ropero AB, Fuentes E, Rovira JM, Ripoll C, Soria B, Nadal A. Non-genomic actions of 17beta-oestradiol in mouse pancreatic beta-cells are mediated by a cGMP-dependent protein kinase. J Physiol Lond. 1999;521(Pt 2):397–407.
Soriano S, Ropero AB, Alonso-Magdalena P, Ripoll C, Quesada I, Gassner B, et al. Rapid regulation of KATP channel activity by 17β-estradiol in pancreatic β-cells involves the estrogen receptor β and the atrial natriuretic peptide receptor. Mol Endocrinol. 2009;23(12):1973–82.
Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34(3):309–38.
Alonso-Magdalena P, Ropero AB, Soriano S, García-Arévalo M, Ripoll C, Fuentes E, et al. Bisphenol-A acts as a potent estrogen via non-classical estrogen triggered pathways. Mol Cell Endocrinol. 2012;355(2):201–7.
Nadal A, Alonso-Magdalena P, Soriano S, Quesada I, Ropero AB. The pancreatic beta-cell as a target of estrogens and xenoestrogens: implications for blood glucose homeostasis and diabetes. Mol Cell Endocrinol. 2009;304(1–2):63–8.
Schuit FC, In't Veld PA, Pipeleers DG. Glucose stimulates proinsulin biosynthesis by a dose-dependent recruitment of pancreatic beta cells. Proc Natl Acad Sci U S A. 1988;85(11):3865–9.
Hasnain SZ, Prins JB, McGuckin MA. Oxidative and endoplasmic reticulum stress in β-cell dysfunction in diabetes. J Mol Endocrinol. 2016;56(2):R33–54.
Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29(1):42–61.
Kuo C-C, Moon K, Thayer KA, Navas-Acien A. Environmental chemicals and type 2 diabetes: an updated systematic review of the epidemiologic evidence. Curr Diab Rep. 2013;13(6):831–49.
Pi J, Bai Y, Zhang Q, Wong V, Floering LM, Daniel K, et al. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes. 2007;56(7):1783–91.
Bodin J, Bølling AK, Samuelsen M, Becher R, Løvik M, Nygaard UC. Long-term bisphenol A exposure accelerates insulitis development in diabetes-prone NOD mice. Immunopharmacol Immunotoxicol. 2013;35(3):349–58.
Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014;6(1):a009191.
Wang T, Li M, Chen B, Xu M, Xu Y, Huang Y, et al. Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J Clin Endocrinol Metab. 2012;97(2):E223–7.
Beydoun HA, Khanal S, Zonderman AB, Beydoun MA. Sex differences in the association of urinary bisphenol-A concentration with selected indices of glucose homeostasis among U.S. adults. Ann Epidemiol. 2014;24(2):90–7.
Pearson JF, Bachireddy C, Shyamprasad S, Goldfine AB, Brownstein JS. Association Between fine particulate matter and diabetes prevalence in the U.S. Dia Care. American Diabetes Association. 2010;33(10):2196–201.
Cranmer M. Exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is associated with hyperinsulinemia and insulin resistance. Toxicol Sci. 2000;56(2):431–6.
Stahlhut RW, van Wijngaarden E, Dye TD, Cook S, Swan SH. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ Health Perspect. 2007;115(6):876–82.
James-Todd TM, Meeker JD, Huang T, Hauser R, Ferguson KK, Rich-Edwards JW, et al. Pregnancy urinary phthalate metabolite concentrations and gestational diabetes risk factors. Environ Int. 2016;96:118–26.
Rajesh P, Balasubramanian K. Phthalate exposure in utero causes epigenetic changes and impairs insulin signalling. J Endocrinol. 2014;223(1):47–66.
Shulman GI, Rothman DL, Jue T, Stein P, DeFronzo RA, Shulman RG. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med. 1990;322(4):223–8.
•• Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, et al. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol. 2016. Recent comprehensive review of metabolism disrupting chemicals.
Ruzzin J, Petersen R, Meugnier E, Madsen L, Lock E-J, Lillefosse H, et al. Persistent organic pollutant exposure leads to insulin resistance syndrome. Environ Health Perspect. 2010 Apr;118(4):465–71.
García-Arévalo M, Alonso-Magdalena P, Rebelo Dos Santos J, Quesada I, Carneiro EM, Nadal A. Exposure to bisphenol-A during pregnancy partially mimics the effects of a high-fat diet altering glucose homeostasis and gene expression in adult male mice. PLoS One. 2014;9(6):e100214.
Jayashree S, Indumathi D, Akilavalli N, Sathish S, Selvaraj J, Balasubramanian K. Effect of bisphenol-A on insulin signal transduction and glucose oxidation in liver of adult male albino rat. Environ Toxicol Pharmacol. 2013;35(2):300–10.
Beischlag TV, Luis Morales J, Hollingshead BD, Perdew GH. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr. 2008;18(3):207–50.
Seefeld MD, Corbett SW, Keesey RE, Peterson RE. Characterization of the wasting syndrome in rats treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 1984;73(2):311–22.
Warner M, Mocarelli P, Brambilla P, Wesselink A, Samuels S, Signorini S, et al. Diabetes, metabolic syndrome, and obesity in relation to serum dioxin concentrations: the Seveso women’s health study. Environ Health Perspect. 2013;121(8):906–11.
Kern PA, Said S, Jackson WG, Michalek JE. Insulin sensitivity following agent orange exposure in Vietnam veterans with high blood levels of 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Clin Endocrinol Metab. 2004;89(9):4665–72.
Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol Nature Publishing Group. 2008;9(5):367–77.
Janesick AS, Blumberg B. Obesogens: an emerging threat to public health. Am J Obstet Gynecol. 2016;214(5):559–65.
Agarwal AK, Garg A. A novel heterozygous mutation in peroxisome proliferator-activated receptor-gamma gene in a patient with familial partial lipodystrophy. J Clin Endocrinol Metab. 2002;87(1):408–11.
Desvergne B, Feige JN, Casals-Casas C. PPAR-mediated activity of phthalates: a link to the obesity epidemic? Mol Cell Endocrinol. 2009;304(1–2):43–8.
Grün F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology Endocrine Society. 2006;147(6):s50–5.
Neel BA, Brady MJ, Sargis RM. The endocrine disrupting chemical tolylfluanid alters adipocyte metabolism via glucocorticoid receptor activation. Mol Endocrinol. 2013;27(3):394–406.
Nieman LK, Biller BMK, Findling JW, Newell-Price J, Savage MO, Stewart PM, et al. The diagnosis of Cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:1526–40.
van Herpen NA, Schrauwen-Hinderling VB. Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol Behav. 2008;94(2):231–41.
Hotamisligil GS, Shargill NS. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91.
de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett. 2008;582(1):97–105.
Yitshak Sade M, Kloog I, Liberty IF, Schwartz J, Novack V. The association between air pollution exposure and glucose and lipids levels. J Clin Endocrinol Metab. 2016;101(6):2460–7.
Kim MJ, Pelloux V, Guyot E, Tordjman J, Bui L-C, Chevallier A, et al. Inflammatory pathway genes belong to major targets of persistent organic pollutants in adipose cells. Environ Health Perspect. 2012;120(4):508–14.
Alonso-Magdalena P, Quesada I, Nadal A. Prenatal exposure to BPA and offspring outcomes: the diabesogenic behavior of BPA. Dose Response. 2015;13(2):1559325815590395.
Wei J, Lin Y, Li Y, Ying C, Chen J, Song L, et al. Perinatal exposure to bisphenol A at reference dose predisposes offspring to metabolic syndrome in adult rats on a high-fat diet. Endocrinology. 2011;152(8):3049–61.
Ryan KK, Haller AM, Sorrell JE, Woods SC, Jandacek RJ, Seeley RJ. Perinatal exposure to bisphenol-a and the development of metabolic syndrome in CD-1 mice. Endocrinology. 2010;151(6):2603–12.
Paul DS, Walton FS, Saunders RJ, Stýblo M. Characterization of the impaired glucose homeostasis produced in C57BL/6 mice by chronic exposure to arsenic and high-fat diet. Environ Health Perspect. 2011;119(8):1104–9.
Longo VD, Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metabolism. 2016.
Beydoun HA, Beydoun MA, Jeng HA, Zonderman AB, Eid SM. Bisphenol-A and sleep adequacy among adults in the National Health and Nutrition Examination Surveys. Sleep. 2016;39(2):467–76.
Weber DN, Hoffmann RG, Hoke ES, Tanguay RL. Bisphenol A exposure during early development induces sex-specific changes in adult zebrafish social interactions. J Toxicol Environ Health Part A. 2015;78(1):50–66.
• Rhee J-S, Kim B-M, Lee B-Y, Hwang U-K, Lee YS, Lee J-S. Cloning of circadian rhythmic pathway genes and perturbation of oscillation patterns in endocrine disrupting chemicals (EDCs)-exposed mangrove killifish Kryptolebias marmoratus. Comp Biochem Physiol C Toxicol Pharmacol. 2014;164:11–20. This study demonstrates the links between MDCs and circadian biology.
Pearson ER, Flechtner I, Njølstad PR, Malecki MT, Flanagan SE, Larkin B, et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med. 2006;355(5):467–77.
Grossman SL, Lessem J. Mechanisms and clinical effects of thiazolidinediones. Expert Opin Investig Drugs. 1997;6(8):1025–40.
Joseph GY, Javorschi S, Hevener AL. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002.
Boden G, Homko C, Mozzoli M, Showe LC, Nichols C, Cheung P. Thiazolidinediones upregulate fatty acid uptake and oxidation in adipose tissue of diabetic patients. Diabetes. 2005;54(3):880–5.
de Souza CJ, Eckhardt M, Gagen K, Dong M, Chen W, Laurent D, et al. Effects of pioglitazone on adipose tissue remodeling within the setting of obesity and insulin resistance. Diabetes American Diabetes Association. 2001;50(8):1863–71.
Hill DS, Wlodarczyk BJ, Mitchell LE, Finnell RH. Arsenate-induced maternal glucose intolerance and neural tube defects in a mouse model. Toxicol Appl Pharmacol. 2009;239(1):29–36.
Acknowledgments
This work was supported by the National Institutes of Health (T32DK007011 to M.S.M.), the American Diabetes Association (Junior Faculty Development Award 1-17-JDF-033 to R.M.S.), the Ministerio de Economia y Competitividad (SAF2014-58335-P to A.N.), and Generalitat Valenciana (PROMETEOII/2015/016 to A.N.). CIBERDEM is an initiative of the Instituto de Salud Carlos III.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Mizuho S. Mimoto and Angel Nadal declare that they have no conflict of interest.
Robert M. Sargis reports honoraria from CVS Health.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Mechanisms of Toxicity
Rights and permissions
About this article
Cite this article
Mimoto, M.S., Nadal, A. & Sargis, R.M. Polluted Pathways: Mechanisms of Metabolic Disruption by Endocrine Disrupting Chemicals. Curr Envir Health Rpt 4, 208–222 (2017). https://doi.org/10.1007/s40572-017-0137-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40572-017-0137-0