Abstract
Purpose of review
Peptide immunotherapy with synthetic peptide immuno-regulatory epitopes (SPIREs) is a safer alternative to conventional allergen immunotherapy (AIT) owing to the reduced risk of IgE-mediated adverse reactions and shorter treatment schedules. Over the last 5 years, significant advances have been made in evaluating SPIREs for the treatment of allergic rhinitis. This review includes a summary of recent phase II and III clinical trials evaluating the efficacy of SPIREs and a brief discussion on their mechanism of action.
Recent findings
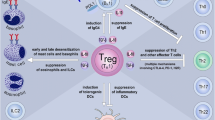
To date, SPIREs have been developed for cat, house dust mite (HDM), ragweed, and grass. Phase II clinical trials showed strong reductions in allergic symptoms, with clinical benefit persisting for up to 2 years after treatment initiation. However, subsequent phase III studies of cat and HDM peptides did not meet their primary endpoint due to significant placebo responses. Mechanistic studies reported a shift from effector T helper 2 (Th2) cells to Th1 and regulatory T cells along with reductions in Th2 cytokines and Th2-associated genes in blood immune cells.
Summary
Peptide immunotherapy with SPIREs is efficacious in reducing symptoms after only 12–14 weeks of treatment. Symptom relief can last up to 2 years after treatment and requires fewer injections compared to subcutaneous AIT. Revised trial designs should be considered to re-evaluate these potentially useful therapies in phase III evaluations to overcome placebo responses.
Similar content being viewed by others
References and recommended reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bantz SK, Zhu Z, Zheng T. The Atopic March: Progression from Atopic Dermatitis to Allergic Rhinitis and Asthma. J Clin Cell Immunol. 2014;5(2):202. https://doi.org/10.4172/2155-9899.1000202.
Noon L. Prophylactic inoculation against hay fever. Lancet. 1911;177(4580):1572–3. https://doi.org/10.1016/S0140-6736(00)78276-6.
Dhami S, Nurmatov U, Arasi S, et al. Allergen immunotherapy for allergic rhinoconjunctivitis: A systematic review and meta-analysis. Allergy. 2017;72(11):1597–631. https://doi.org/10.1111/all.13201.
Dhami S, Kakourou A, Asamoah F, et al. Allergen immunotherapy for allergic asthma: A systematic review and meta-analysis. Allergy. 2017;72(12):1825–48. https://doi.org/10.1111/all.13208.
Curin M, Garib V, Valenta R. Single recombinant and purified major allergens and peptides: How they are made and how they change allergy diagnosis and treatment. Ann Allergy Asthma Immunol. 2017;119(3):201–9. https://doi.org/10.1016/j.anai.2016.11.022.
Canonica GW, Bousquet J, Casale T, et al. Sub-lingual immunotherapy: World Allergy Organization Position Paper 2009. Allergy. 2009;64(Suppl 91):1–59. https://doi.org/10.1111/j.1398-9995.2009.02309.x.
Epstein TG, Liss GM, Murphy-Berendts K, Bernstein DI. Risk factors for fatal and nonfatal reactions to subcutaneous immunotherapy: National surveillance study on allergen immunotherapy (2008–2013). Ann Allergy Asthma Immunol. 2016;116(4):354–359.e2. https://doi.org/10.1016/j.anai.2016.02.001.
Norman PS, Ohman JL, Long AA, et al. Treatment of cat allergy with T-cell reactive peptides. Am J Respir Crit Care Med. 1996;154(6 Pt 1):1623–8. https://doi.org/10.1164/ajrccm.154.6.8970345.
Creticos PS, Hebert J, Philip G, Group, A.R.S. Efficacy of Allervax® ragweed peptides in the treatment of ragweed-induced allergy. J Allergy Clin Immunol. 1997;99:S401:A1631.
Creticos PS. Advances in synthetic peptide immuno-regulatory epitopes. World Allergy Organ J. 2014;7:71. https://doi.org/10.1186/1939-4551-7-30.
O’Hehir RE, Prickett SR, Rolland JM. T Cell Epitope Peptide Therapy for Allergic Diseases. Curr Allergy Asthma Rep. 2016;16(2):14. https://doi.org/10.1007/s11882-015-0587-0.
•• Patel D, Couroux P, Hickey P, et al. Fel d 1-derived peptide antigen desensitization shows a persistent treatment effect 1 year after the start of dosing: a randomized, placebo-controlled study. J Allergy Clin Immunol. 2013;131(1):103–109.e1–7. https://doi.org/10.1016/j.jaci.2012.07.028. Evaluates the clinical efficacy of Cat-PAD in cat-allergic patients one year after treatment initiation.
•• Couroux P, Patel D, Armstrong K, Larché M, Hafner RP. Fel d 1-derived synthetic peptide immuno-regulatory epitopes show a long-term treatment effect in cat allergic subjects. Clin Exp Allergy. 2015;45(5):974–81. https://doi.org/10.1111/cea.12488. Follow-up study evaluating a possible treatment effect of Cat-PAD two years after treatment initiation using an Environmental Exposure Chamber.
Circassia Announces Top-Line Results from Cat Allergy Phase III Study – Press Releases [Internet]. Circassia. Available at: http://www.circassia.com/media/press-releases/circassia-announces-top-line-results-from-cat-allergy-phase-iii-study/. Accessed March 3, 2018.
• Hafner R, Couroux P, Armstrong K, Salapatek A, Patel D, Larche M. Persistent Treatment Effect Achieved At One Year After Four Doses Of Der p Derived Synthetic Peptide Immuno-Regulatory Epitopes In An Exposure Chamber Model Of House Dust Mite Allergy. J Allergy Clin Immunol. 2014;133(2):AB289. https://doi.org/10.1016/j.jaci.2013.12.1023. Evaluates the clinical efficacy of HDM-PAD in HDM-allergic patients one year after treatment initiation.
Hafner R, Salapatek A, Larché M, Ahenkorah B, Patel P, Pawsey S. Initial Evidence of Sustained Efficacy of House Dust Mite Synthetic Peptide Immuno Regulatory Epitopes 2 Years after a Short Course of Treatment in House Dust Mite (HDM) Allergic Subjects. J Allergy Clin Immunol. 2015;135(2):AB142. https://doi.org/10.1016/j.jaci.2014.12.1399.
Circassia Announces Top-Line Results from House Dust Mite Allergy Field Study – Press Releases [Internet]. Circassia. Available from: http://www.circassia.com/media/press-releases/circassia-announces-top-line-results-from-house-dust-mite-allergy-field-study/. Accessed March 3, 2018.
•• Hafner RP, Salapatek A, Patel D, Larché M, Laidler P. Validation of Peptide Immunotherapy as a New Approach in the Treatment of Allergic Rhinoconjunctivitis: The Clinical Benefits of Treatment with Amb a 1 Derived T cell Epitopes. J Allergy Clin Immunol. 2012;129(2):AB368. https://doi.org/10.1016/j.jaci.2012.01.017. Study evaluates the efficacy of SPIREs derived from the major ragweed allergen Amb a 1 in ragweed-allergic patients.
Circassia Announces Top-Line Results from Ragweed Allergy Treatment Phase IIb Chamber Study – Press Releases [Internet]. Circassia. Available from: http://www.circassia.com/media/press-releases/circassia-announces-top-line-results-from-ragweed-allergy-treatment-phase-iib-chamber-study/. Accessed March 3, 2018.
•• Ellis AK, Frankish CW, O’Hehir RE, et al. Treatment with grass allergen peptides improves symptoms of grass pollen-induced allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2017;140(2):486–96. https://doi.org/10.1016/j.jaci.2016.11.043. Describes a study evaluating the efficacy of a pre-seasonal treatment course of Grass-SPIRE on grass-induced AR symptoms.
• Ellis A, Frankish CW, Armstrong K, et al. Persistent Treatment Effect with Grass Synthetic Peptide Immuno-Regulatory Epitopes in Grass Allergy Symptoms in an Environmental Exposure Unit Challenge after a Second Season of Natural Pollen Exposure. J Allergy Clin Immunol. 2015;135(2):AB158. https://doi.org/10.1016/j.jaci.2014.12.1457. Optional follow-up study re-evaluating treatment efficacy of Grass-SPIRE in grass-allergic patients one year after treatment initiation in an Environmental Exposure Unit.
Ellis A, Frankish C, Armstrong K, et al. Persistent Treatment Effect with Grass Synthetic Peptide Immuno-Regulatory Epitopes on Grass Allergy Symptoms in the Environmental Exposure Unit after a Third Season of Natural Exposure. Allergy. 2015;70(Suppl S101):A81. https://doi.org/10.1111/all.12715.
• Worm M, Lee H-H, Kleine-Tebbe J, et al. Development and preliminary clinical evaluation of a peptide immunotherapy vaccine for cat allergy. J Allergy Clin Immunol. 2011;127(1):89–97, 97.e1–14. https://doi.org/10.1016/j.jaci.2010.11.029. Provides detail on the in vitro assays used to identify and validate SPIREs derived from the major cat allergen Fel d 1. Study also evaluates the safety of Cat-PAD.
Pawsey S, Hafner RP, Casale TB, et al. Safety, Tolerability and Efficacy of Cat-Peptide Antigen Desensitisation (Cat-PAD) in Cat-Allergic Children – Findings from a Pilot Study. J Allergy Clin Immunol. 2017;139(2):AB256. https://doi.org/10.1016/j.jaci.2016.12.823.
Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(1 Suppl):S1–55. https://doi.org/10.1016/j.jaci.2010.09.034.
Hickey PLC, Cheema AS, Larche M, Hafner R. Safety and Tolerability of Fel d 1-Derived Peptide Antigen Desensitization in Subjects with Controlled Asthma. J Allergy Clin Immunol. 2013;131(2):AB206. https://doi.org/10.1016/j.jaci.2012.12.1404.
Pawsey S, Patel D, Hafner R, Hickey PLC, Powell J. Safety of House Dust Mite Synthetic Peptide Immuno-Regulatory Epitopes in Patients with House Dust Mite Allergy and Controlled Asthma. J Allergy Clin Immunol. 2015;135(2):AB142. https://doi.org/10.1016/j.jaci.2014.12.1400.
Narkus A, Lehnigk U, Haefner D, Klinger R, Pfaar O, Worm M. The placebo effect in allergen-specific immunotherapy trials. Clin Transl Allergy. 2013;3:42. https://doi.org/10.1186/2045-7022-3-42.
• Ellis AK, North ML, Walker T, Steacy LM. Environmental exposure unit: a sensitive, specific, and reproducible methodology for allergen challenge. Ann Allergy Asthma Immunol. 2013;111(5):323–8. https://doi.org/10.1016/j.anai.2013.07.019. Provides a comprehensive description of the Environmental Exposure Unit as a useful model to evaluate the clinical efficacy of new AR medications.
North ML, Soliman M, Walker T, Steacy LM, Ellis AK. Controlled Allergen Challenge Facilities and Their Unique Contributions to Allergic Rhinitis Research. Curr Allergy Asthma Rep. 2015;15(4):11. https://doi.org/10.1007/s11882-015-0514-4.
Day JH, Horak F, Briscoe MP, et al. The role of allergen challenge chambers in the evaluation of anti-allergic medication: an international consensus paper. Clin Exp Allergy Rev. 2006;6(2):31–59. https://doi.org/10.1111/j.1365-2222.2005.00099.x.
Cox LS, Larenas Linnemann D, Nolte H, Weldon D, Finegold I, Nelson HS. Sublingual immunotherapy: a comprehensive review. J Allergy Clin Immunol. 2006;117(5):1021–35. https://doi.org/10.1016/j.jaci.2006.02.040.
Rodríguez Del Río P, Vidal C, Just J, et al. The European Survey on Adverse Systemic Reactions in Allergen Immunotherapy (EASSI): A paediatric assessment. Pediatr Allergy Immunol. 2017;28(1):60–70. https://doi.org/10.1111/pai.12660.
Calderón MA, Vidal C, Rodríguez Del Río P, et al. European Survey on Adverse Systemic Reactions in Allergen Immunotherapy (EASSI): a real-life clinical assessment. Allergy. 2017;72(3):462–72. https://doi.org/10.1111/all.13066.
Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J Allergy Clin Immunol. 2014;133(3):621–31. https://doi.org/10.1016/j.jaci.2013.12.1088.
Larché M. Regulatory T cells in allergy and asthma. Chest. 2007;132(3):1007–14. https://doi.org/10.1378/chest.06-2434.
Shamji MH, Ljørring C, Francis JN, et al. Functional rather than immunoreactive levels of IgG4 correlate closely with clinical response to grass pollen immunotherapy. Allergy. 2012;67(2):217–26. https://doi.org/10.1111/j.1398-9995.2011.02745.x.
James LK, Shamji MH, Walker SM, et al. Long-term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. J Allergy Clin Immunol. 2011;127(2):509–516.e1–5. https://doi.org/10.1016/j.jaci.2010.12.1080.
Akdis CA, Akdis M. Mechanisms of immune tolerance to allergens: role of IL-10 and Tregs. J Clin Invest. 2014;124(11):4678–80. https://doi.org/10.1172/JCI78891.
Böhm L, Maxeiner J, Meyer-Martin H, et al. IL-10 and regulatory T cells cooperate in allergen-specific immunotherapy to ameliorate allergic asthma. J Immunol. 2015;194(3):887–97. https://doi.org/10.4049/jimmunol.1401612.
van de Veen W, Stanic B, Yaman G, et al. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol. 2013;131(4):1204–12. https://doi.org/10.1016/j.jaci.2013.01.014.
Oldfield WL, Kay AB, Larché M. Allergen-derived T cell peptide-induced late asthmatic reactions precede the induction of antigen-specific hyporesponsiveness in atopic allergic asthmatic subjects. J Immunol. 2001;167(3):1734–9. https://doi.org/10.4049/jimmunol.167.3.1734.
Smith TRF, Alexander C, Kay AB, Larché M, Robinson DS. Cat allergen peptide immunotherapy reduces CD4(+) T cell responses to cat allergen but does not alter suppression by CD4(+) CD25(+) T cells: a double-blind placebo-controlled study. Allergy. 2004;59(10):1097–101. https://doi.org/10.1111/j.1398-9995.2004.00601.x.
Verhoef A, Alexander C, Kay AB, Larché M. T cell epitope immunotherapy induces a CD4+ T cell population with regulatory activity. PLoS Med. 2005;2(3):e78. https://doi.org/10.1371/journal.pmed.0020078.
Tarzi M, Klunker S, Texier C, et al. Induction of interleukin-10 and suppressor of cytokine signalling-3 gene expression following peptide immunotherapy. Clin Exp Allergy. 2006;36(4):465–74. https://doi.org/10.1111/j.1365-2222.2006.02469.x.
• Alexander C, Ying S, B Kay A, Larché M. Fel d 1-derived T cell peptide therapy induces recruitment of CD4+ CD25+; CD4+ interferon-gamma+ T helper type 1 cells to sites of allergen-induced late-phase skin reactions in cat-allergic subjects. Clin Exp Allergy. 2005;35(1):52–8. https://doi.org/10.1111/j.1365-2222.2005.02143.x. Provides support for a possible role of CD4 + CD25+ and CD4 + IFN-γ + T cells in the tolerance-inducing properties of Fel d 1-derived SPIREs in cat-allergic patients.
• Campbell JD, Buckland KF, McMillan SJ, et al. Peptide immunotherapy in allergic asthma generates IL-10-dependent immunological tolerance associated with linked epitope suppression. J Exp Med. 2009;206(7):1535–47. https://doi.org/10.1084/jem.20082901. Illustrates the concept of linked epitope suppression and the role of IL-10 in this process.
Soliman M, North ML, Steacy LM, Thiele J, Adams DE, Ellis AK. Nasal allergen challenge studies of allergic rhinitis: a guide for the practicing clinician. Ann Allergy Asthma Immunol. 2014;113(3):250–6. https://doi.org/10.1016/j.anai.2014.06.018.
Gliddon DR, Kim YW, Shannon CP, et al. Whole blood immune transcriptome profiling reveals systemic pathways associated with the mechanism of action of cat-synthetic peptide immunoregulatory epitopes. Allergy. 2015;70(Suppl S101):A1747. https://doi.org/10.1111/all.12724.
Tonti E, Gliddon DR, Kim YW, et al. RNA-seq transcriptome analysis of human allergen-specific T cells suggests a role for IL-8 downregulation and modulation of allergic inflammatory cells as one of several potential mechanisms of action of intradermal immunotherapy with Fel d 1 synthetic peptides. Allergy. 2016;71(Suppl S102):A4. https://doi.org/10.1111/all.12970.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
AKE has participated in advisory boards for ALK Abello, Circassia Ltd., GlaxoSmithKline, Johnson & Johnson, Merck and Novartis, and has been a speaker for Aralez, AstraZeneca, Boheriger Ingelheim, Meda, Mylan, Merck, Novartis, Pediapharm, Pfizer, and Takeda. Her institution has received research grants from Circassia Ltd., Green Cross Pharmaceuticals, GlaxoSmithKline, Sanofi, Sun Pharma, Merck, Novartis and Pfizer. AKE is also a consultant to Bayer Inc. MWT has no relevant conflicts of interest to disclose.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Specific Immunotherapy
Rights and permissions
About this article
Cite this article
Tenn, M.W., Ellis, A.K. Mechanism of Synthetic Peptide Immuno-Regulatory Epitopes and Their Clinical Efficacy in the Treatment of Allergic Disease. Curr Treat Options Allergy 5, 291–301 (2018). https://doi.org/10.1007/s40521-018-0177-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40521-018-0177-1