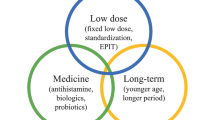
Opinion statement
The current standard of care treatment for food allergy relies on avoidance. However, as outlined in this review, over the past decade, the literature has grown significantly showing promising results for interventional therapies for IgE-mediated food allergies utilizing oral immunotherapy (OIT), sublingual immunotherapy (SLIT), and epicutaneous immunotherapy (EPIT). Within the near future, we expect to have multiple FDA-approved products on the market for treatment of life-threatening food allergies and will have options to present to our patients. As we move into this next phase of our practice, we will need to consider meaningful endpoints for our patients (e.g., likelihood of inducing desensitization versus sustained unresponsiveness, the relevance of various threshold changes induced by therapy, effects on quality of life, etc.). This review provides a comprehensive overview of the currently published literature and will prepare the practicing allergist for a thoughtful discussion with their patients and colleagues on the most recent developments in the field.
Similar content being viewed by others
References and Recommended Readings
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Branum AM, Lukacs SL. Food allergy among children in the United States. Pediatrics. 2009;125:1549–55.
Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis and treatment. J Allergy Clin Immunol. 2014;133:291–307.
Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2011;127:594–602.
Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel report. Nutr Res. 2011;31(1):61–75.
Primeau MN, Kagan R, Joseph L, et al. The psychological burden of peanut allergy as perceived by adults with peanut allergy and the parents of peanut-allergic children. Clin Exp Allergy. 2000;30:1135–43.
Sicherer SH, Noone SA, Munoz-Furlong A. The impact of childhood food allergy on quality of life. Ann Allergy Asthma Immunol. 2001;87:461–4.
Robbins KA, Wood RA, Keet CA. Milk allergy is associated with decreased growth in US children. J Allergy Clin Immunol. 2014;134:1466–8.e6.
Freier S, Kletter B. Milk allergy in infants and young children. Current knowledge Clin Pediatr. 1970;9:449–54.
Schofield AT. A case of egg poisoning. Lancet. 1908;1:716.
Schloss OM. A case of allergy to common foods. Am J Dis Child. 1912;3:341.
Schloss OM. Allergy in infants and children. Am J Dis Child. 1920;19:433–55.
Freeman J. “Rush” inoculation, with special reference to hay-fever treatment. Lancet. 1930;215:744–7.
Deol S, Bird JA. Current opinion and review on peanut oral immunotherapy. Hum Vaccin Immunother. 2014;10(10):3017–21.
Jones SM, Pons L, Roberts JL, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124(2):292–300. 300.e1-97
• Vickery BP, Scurlock AM, Kulis M, et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol. 2014; 133(2):468–75. Long-term immune modulation for patients undergoing OIT is largely unknown. Vickery et al. report the effects of peanut OIT after years of therapy.
•• Varshney P, Jones SM, Scurlock AM, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011;127:654–60. This is the first randomized, placebo-controlled trial demonstrating the efficacy of peanut OIT
Anagnostou K, Clark A, King Y, et al. Efficacy and safety of high-dose peanut oral immunotherapy with factors predicting outcome. Clin Exp Allergy. 2011;41:1273–81.
Anagnostou K, Islam S, King Y, et al. Assessing the efficacy of oral immunotherapy for the desensitisation of peanut allergy in children (STOP II): a phase 2 randomised control trial. Lancet. 2014;383:1297–304.
Blumchen K, Ulbricht H, Staden U, et al. Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol. 2010;126(1):83–91.
Burks AW, Jones SM, Wood RA, et al. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med. 2012;367:233–43.
Jones SM, Burks AW, Keet C, et al. Long-term treatment with egg oral immunotherapy enhances sustained unresponsiveness that persists after cessation of therapy. J Allergy Clin Immunol. 2016;137:1117–27.
Meglio P, Bartone E, Plantamura M, et al. A protocol for oral desensitization in children with IgE-mediated cow’s milk allergy. Allergy. 2004;59:980–7.
Longo G, Barbi E, Berti I, et al. Specific oral tolerance induction in children with very severe cow’s milk-induced reactions. J Allergy Clin Immunol. 2008;121:343–7.
Skripak JM, Nash SD, Rowley H, Brereton NH, Oh S, Hamilton RG, et al. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow’s milk allergy. J Allergy Clin Immunol. 2008;122:1154–60.
Narisety SD, Skripak JM, Steele P, et al. Open-label maintenance after milk oral immunotherapy for IgE-mediated cow’s milk allergy. J Allergy Clin Immunol. 2009;124(3):610–2.
Sicherer S, Munoz-Furlong A, Sampson H. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. J Allergy Clin Immunol. 2003;112:1203–7.
Hofmann AM, Scurlock AM, Jones SM, et al. Safety of a peanut oral immunotherapy protocol in children with peanut allergy. J Allergy Clin Immunol. 2009;124(2):286–91. 291.e1-6
Lemon-Mulé H, Sampson HA, Sicherer SH, et al. Immunologic changes in children with egg allergy ingesting extensively heated egg. J Allergy Clin Immunol. 2008;122(5):977–83. e1
Vickery BP, Pons L, Kulis M, et al. Individualized IgE-based dosing of egg oral immunotherapy and the development of tolerance. Ann Allergy Asthma Immunol. 2010;105:444–50.
Sicherer SH, Sampson HA. Cow’s milk protein-specific IgE concentrations in two age groups of milk-allergic children and in children achieving clinical tolerance. Clin Exp Allergy. 1999;29(4):507–12.
Host A, Halken S, Jacobsen HP, et al. Clinical course of cow’s milk protein allergy/intolerance and atopic diseases in childhood. Pediatr Allergy Immunol. 2002;13(Suppl 15):23–8.
Staden U, Rolinck-Werninghaus C, Brewe F, Wahn U, Niggemann B, Beyer K. Specific oral tolerance induction in food allergy in children: efficacy and clinical patterns of reaction. Allergy. 2007;62(11):1261–9.
Bégin P, Winterroth LC, Dominguez T, et al. Safety and feasibility of oral immunotherapy to multiple allergens for food allergy. Allergy Asthma Clin Immunol. 2014;10:1.
Nadeau KC, Schneider LC, Hoyte L, et al. Rapid oral desensitization in combination with omalizumab therapy in patients with cow’s milk allergy. J Allergy Clin Immunol. 2011;127(6):1622–4.
Schneider LC, Rachid R, LeBovidge J, et al. A pilot study of omalizumab to facilitate rapid oral desensitization in high-risk peanut-allergic patients. J Allergy Clin Immunol. 2013;132(6):1368–74.
• Wood RA, Kim JS, Lindblad R, et al. A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow’s milk allergy. J Allergy Clin Immunol. 2016;137(4):1103–10.e1-11. Safety concerns are one of the primary barriers to implementing OIT in clinical practice. Wood et al. show improvement in the safety profile of milk OIT with concomitant omalizumab administration; however, desensitization and sustained unresponsiveness were not affected
Bégin P, Dominguez T, Wilson SP, et al. Phase 1 results of safety and tolerability in a rush oral immunotherapy protocol to multiple foods using omalizumab. Allergy Asthma Clin Immunol. 2014;10:7.
Kim EH, Bird JA, Kulis M, et al. Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol. 2011;127(3):640–646.e1.
Fleischer DM, Burks AW, Vickery BP, et al. Sublingual immunotherapy for peanut allergy: a randomized, double-blind, placebo-controlled multicenter trial. J Allergy Clin Immunol. 2013;131(1):119–127.e7.
Narisety SD, Frischmeyer-Guerrerio PA, Keet CA, et al. A randomized, double-blind, placebo-controlled pilot study of sublingual versus oral immunotherapy for the treatment of peanut allergy. J Allergy Clin Immunol. 2015;135(5):1275–82. e1-6
Keet CA, Frischmeyer-Guerrerio PA, Thyagarajan A, et al. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J Allergy Clin Immunol. 2012;129:448–55. e1-5
Dupont C, Kalach N, Soulaines P, et al. Cow’s milk epicutaneous immunotherapy in children: a pilot trial of safety, acceptability, and impact on allergic reactivity [letter]. J Allergy Clin Immunol. 2010;125(5):1165–7.
Jones SM, Agbotounou WK, Fleischer DM, et al. Safety of epicutaneous immunotherapy for the treatment of peanut allergy: a phase 1 study using the Viaskin patch [letter]. J Allergy Clin Immunol. 2016;137(4):1258–61.
Sampson HA. Anaphylaxis and emergency treatment. Pediatrics. 2003;111:1601–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Tiffany J. Lieu declares no conflict of interest.
J. Andrew Bird has received research support from Aimmune Therapeutics, DBV Technologies, and Food Allergy Research and Education. He is on the speaker’s bureau for Nutricia North America, Aimmune Therapeutics, and DBV Technologies. He has received consulting fees from Wedbush Securities.
Human and animal rights and informed consent
With regard to the authors’ research cited in this paper, all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. In addition, all applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
This article is part of the Topical Collection on Specific Immunotherapy
Rights and permissions
About this article
Cite this article
Lieu, T.J., Bird, J.A. Oral Immunotherapy, Sublingual Immunotherapy, or Epicutaneous Immunotherapy: Which Is the Right Solution?. Curr Treat Options Allergy 4, 1–13 (2017). https://doi.org/10.1007/s40521-017-0116-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40521-017-0116-6