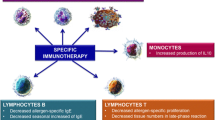
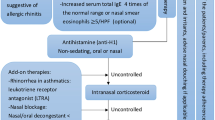
Opinion statement
Current treatment guidelines for allergic rhinitis (AR) recommend a stepwise therapeutic approach that combines patient education with specific allergen avoidance, symptomatic pharmacotherapy, and allergen immunotherapy (AIT). The available pharmacological strategies provide suboptimal symptom relief in patients with moderate-to-severe disease who continue to experience symptoms while treated, even on multiple therapies. The development of new symptomatic drugs with improved pharmacokinetics and safety has recently opened new perspective in the field of AR treatment, overcoming current limitations and increasing compliance to therapy. However, the ultimate research goal is beyond symptomatic treatment, and is mainly directed at modifying the immune response to allergens. In this direction, promising advances are expected in the fields of AIT and biological drugs. Significant research efforts are also focused on new therapeutic targets involved in the Th2-driven pathway inflammation.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bousquet J et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update. Allergy. 2008;63 Suppl 86:8–160.
Roberts G et al. Paediatric rhinitis: position paper of the European Academy of Allergy and Clinical Immunology. Allergy. 2013;68:1102–16. This study provides a complete overview of rhinitis in children, with particular attention to the differential diagnosis.
Wheatley LM, Togias A. Clinical practice. Allergic rhinitis. N Engl J Med. 2015;372:456–63.
Meltzer EO, Bukstein DA. The economic impact of allergic rhinitis and current guidelines for treatment. Ann Allergy Asthma Immunol. 2011;106:S12–6.
Licari A et al. Current recommendations and emerging options for the treatment of allergic rhinitis. Expert Rev Clin Immunol. 2014;10:1337–47.
Bousquet J et al. Costs associated with persistent allergic rhinitis are reduced by levocetirizine. Allergy. 2005;60:788–94.
Ciprandi G et al. Patient-related factors in rhinitis and asthma: the satisfaction with allergy treatment survey. Curr Med Res Opin. 2011;27:1005–11.
Bousquet J et al. Unmet needs in severe chronic upper airway disease (SCUAD). J Allergy Clin Immunol. 2009;124:428–33.
Nurmatov U, van Schayck CP, Hurwitz B, Sheikh A. House dust mite avoidance measures for perennial allergic rhinitis: an updated Cochrane systematic review. Allergy. 2012;67:158–65.
Licari A et al. Emerging drugs for the treatment of perennial allergic rhinitis. Expert Opin Emerg Drugs. 2016;21:57–67.
Brozek JL et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466–76. This study provides a new methodological approach (grading of recommendation) in updating allergic rhinitis and its impact on asthma guidelines.
Church MK et al. Risk of first generation H1-antihistamines: a GA2LEN position paper. Allergy. 2010;65:459–66.
Weiner JM, Abramson MJ, Puy RM. Intranasal corticosteroids versus oral H1 receptor antagonists in allergic rhinitis: systematic review of randomized controlled trials. BMJ. 1998;317:1624–9.
Greiner A, Hellings P, Rotiroti G, Scadding G. Allergic rhinitis. Lancet. 2011;378:2112–22.
Berger WE, Meltzer EO. Intranasal spray medications for maintenance therapy of allergic rhinitis. Am J Rhinol Allergy. 2015;29:273–82.
Corren J. Intranasal corticosteroids for allergic rhinitis. How do different agents compare? J Allergy Clin Immunol. 1999:S144-9.
Wolthers OD. Impact of inhaled and intranasal corticosteroids on the growth of children. Bio Drugs. 2000;13:347–57.
Nielsen LP, Mygind N, Dahl R. Intranasal corticosteroids for allergic rhinitis: superior relief? Drugs. 2001;61:1563–79.
Patel P et al. Randomized, double-blind, placebo-controlled study of montelukast for treating perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2005;95:551–7.
Chen ST et al. Randomized placebo-controlled trial comparing montelukast and cetirizine for treating perennial allergic rhinitis in children aged 2–6 yr. Pediatr Allergy Immunol. 2006;17:49–54.
Yilmaz O, Altintas D, Rondon C, Cingi C, Oghan F. Effectiveness of montelukast in pediatric patients with allergic rhinitis. Int J Pediatr Otorhinolaryngol. 2013;77:1922–4.
Hellings PW et al. Uncontrolled allergic rhinitis and chronic rhinosinusitis: where do we stand today? Allergy. 2013;68:1–7.
Jutel M et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015;136:556–68.
Jutel M et al. International consensus on allergen immunotherapy II: mechanisms, standardization, and pharmacoeconomics. J Allergy Clin Immunol. 2016;137:358–68.
Ciprandi G et al. From IgE to clinical trials of allergic rhinitis. Expert Rev Clin Immunol. 2015;11:1321–33. This reference is of interest as it provides an update on current findings on immunological and clinical effects of allergen immunotherapy and anti-IgE therapy in allergic rhinitis.
Burks AW et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131:1288–96.e3.
Nelson HS. Subcutaneous immunotherapy versus sublingual immunotherapy: which is more effective? J Allergy Clin Immunol Pract. 2014;2:144–9.
Malling HJ, Bousquet J. Subcutaneous immunotherapy for allergic rhinoconjunctivitis, allergic asthma, and prevention of allergic diseases. Clin Allergy Immunol. 2008;21:343–58.
Wilson DR, Lima MT, Durham SR. Sublingual immunotherapy for allergic rhinitis: systematic review and meta-analysis. Allergy. 2005;60:4–12.
Canonica GW et al. Sub-lingual immunotherapy: World Allergy Organization Position Paper 2009. Allergy. 2009;64:1–59.
Canonica GW et al. Sublingual immunotherapy: World Allergy Organization position paper 2013 update. World Allergy Organ J. 2014;7:6.
Bousquet J et al. Advances in pharmacotherapy for the treatment of allergic rhinitis; MP29-02 (a novel formulation of azelastine hydrochloride and fluticasone propionate in an advanced delivery system) fills the gaps. Expert Opin Pharmacother. 2015;16:913–28.
Klimek L, Bousquet J, Price D. Safety evaluation of MP29-02 (a novel intranasal formulation of azelastine hydrochloride and fluticasone propionate) for allergic rhinitis. Expert Opin Drug Saf. 2016;15:117–29.
Meltzer E et al. Clinically relevant effect of a new intranasal therapy (MP29-02) in allergic rhinitis assessed by responder analysis. Int Arch Allergy Immunol. 2013;161:369–77.
Price D et al. A new therapy (MP29-02) is effective for the long-term treatment of chronic rhinitis. J Investig Allergol Clin Immunol. 2013;23:495–503.
Berger WE et al. Long-term, randomized safety study of MP29-02 (a novel intranasal formulation of azelastine hydrochloride and fluticasone propionate in an advanced delivery system) in subjects with chronic rhinitis. J Allergy Clin Immunol Pract. 2014;2:179–85.
Klimek L et al. Effectiveness of MP29-02 for the treatment of allergic rhinitis in real-life: results from a noninterventional study. Allergy Asthma Proc. 2015;36:40–7.
Dymista summary of product characteristic. 2015 Available from: https://www.medicines.org.uk/emc/medicine/27579/SPC/Dymista+Nasal+Spray/
Berger W et al. MP-AzeFlu is more effective than fluticasone propionate for the treatment of allergic rhinitis in children. Allergy. 2016;71:1219–22.
Available from: https://clinicaltrials.gov/ct2/show/NCT02498509?term=CKD-342&rank=1
Simons FE, Simons KJ. Histamine and H1-antihistamines: celebrating a century of progress. J Allergy Clin Immunol. 2011;128:1139–1150.e4.
Stokes JR et al. The effects of an H3 receptor antagonist (PF-03654746) with fexofenadine on reducing allergic rhinitis symptoms. J Allergy Clin Immunol. 2012;129:409–12.
Barchuk WT, Salapatek AM, Ge T, D’Angelo P, Liu X. A proof-of-concept study of the effect of a novel H3-receptor antagonist in allergen-induced nasal congestion. J Allergy Clin Immunol. 2013;132:838–46.e1-6.
North ML et al. Add-on histamine receptor-3 antagonist for allergic rhinitis: a double blind randomized crossover trial using the environmental exposure unit. Allergy, Asthma Clin Immunol. 2014;10:33.
Daley-Yates P et al. The efficacy and tolerability of two novel H(1)/H(3) receptor antagonists in seasonal allergic rhinitis. Int Arch Allergy Immunol. 2012;158:84–98.
Avalaible from: http://www.gene.com/download/pdf/xolair_prescribing.pdf
Licari A et al. Omalizumab in children. Paediatr Drugs. 2014;16:491–502.
Holgate S et al. The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation. J Allergy Clin Immunol. 2005;115:459–65.
Prussin C et al. Omalizumab treatment downregulates dendritic cell FcepsilonRI expression. J Allergy Clin Immunol. 2003;112:1147–54.
Holgate S, Smith N, Massanari M, Jimenez P. Effects of omalizumab on markers of inflammation in patients with allergic asthma. Allergy. 2009;64:1728–36.
Kuehr J et al. Efficacy of combination treatment with anti-IgE plus specific immunotherapy in polysensitized children and adolescents with seasonal allergic rhinitis. J Allergy Clin Immunol. 2002;109:274–80.
Casale TB et al. Omalizumab pretreatment decreases acute reactions after rush immunotherapy for ragweed-induced seasonal allergic rhinitis. J Allergy Clin Immunol. 2006;117:134–40.
Kopp MV et al. Combination of omalizumab and specific immunotherapy is superior to immunotherapy in patients with seasonal allergic rhinoconjunctivitis and comorbid seasonal allergic asthma. Clin Exp Allergy. 2009;39:271–9.
Kamin W, Kopp MV, Erdnuess F. Safety of anti-IgE treatment with omalizumab in children with seasonal allergic rhinitis undergoing specific immunotherapy simultaneously. Pediatr Allergy Immunol. 2010;21:e160–5.
Casale TB et al. Use of an anti-IgE humanized monoclonal antibody in ragweed-induced allergic rhinitis. J Allergy Clin Immunol. 1997;100:110–21.
Adelroth E et al. Recombinant humanized mAb-E25, an anti-IgE mAb, in birch pollen-induced seasonal allergic rhinitis. J Allergy Clin Immunol. 2000;106:253–9.
Casale TB et al. Effect of omalizumab on symptoms of seasonal allergic rhinitis: a randomized controlled trial. JAMA. 2001;286:2956–7.
Chervinsky P et al. Omalizumab, an anti-IgE antibody, in the treatment of adults and adolescents with perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2003;91:160–7.
Vignola AM et al. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with concomitant allergic asthma and persistent allergic rhinitis: SOLAR. Allergy. 2004;59:709–17.
Okubo K, Ogino S, Nagakura T, Ishikawa T. Omalizumab is effective and safe in the treatment of Japanese cedar pollen-induced seasonal allergic rhinitis. Allergol Int. 2006;55:379–86.
Nagakura T et al. Omalizumab is more effective than suplatast tosilate in the treatment of Japanese cedar pollen-induced seasonal allergic rhinitis. Clin Exp Allergy. 2008;38:329–37.
Tsabouri S, Tseretopoulou X, Priftis K, Ntzani EE. Omalizumab for the treatment of inadequately controlled allergic rhinitis: a systematic review and meta-analysis of randomized clinical trials. J Allergy Clin Immunol Pract. 2014;2:332–40.e1. A comprehensive review and meta-analysis of clinical trials on omalizumab for the treatment of allergic rhinitis.
Arm JP et al. Pharmacokinetics, pharmacodynamics and safety of QGE031 (ligelizumab), a novel high-affinity anti-IgE antibody, in atopic subjects. Clin Exp Allergy. 2014;44:1371–85.
Chu SY et al. Reduction of total IgE by targeted coengagement of IgE B-cell receptor and FcγRIIb with Fc-engineered antibody. J Allergy Clin Immunol. 2012;129:1102–15.
Liour SS, Tom A, Chan YH, Chang TW. Treating IgE-mediated diseases via targeting IgE-expressing B cells using an anti-CεmX antibody. Pediatr Allergy Immunol. 2016;27:446–51.
Bergmann KC et al. Efficacy and safety of sublingual tablets of house dust mite allergen extracts in adults with allergic rhinitis. J Allergy Clin Immunol. 2014;133:1608–14.
Demoly P et al. Effective treatment of house dust mite-induced allergic rhinitis with 2 doses of the SQ HDM SLIT-tablet: results from a randomized double-blind, placebo-controlled phase III trial. J Allergy Clin Immunol. 2016;137:444–451.e8.
Roux M et al. Efficacy and safety of sublingual tablets of house dust mite allergen extracts: results of a dose-ranging study in an environmental exposure chamber. J Allergy Clin Immunol. 2016;138:451–458.e5.
Maloney J et al. Safety of house dust mite sublingual immunotherapy standardized quality tablet in children allergic to house dust mites. Ann Allergy Asthma Immunol. 2016;116:59–65.
Klimek L, Pfaar O, Worm M. New opportunities for allergen immunotherapy using synthetic peptide immuno-regulatory epitopes (SPIREs). Expert Rev Clin Immunol. 2016;13:1–13.
Worm M. SPIREs: a new horizon for allergic disease treatment? Expert Rev Clin Immunol. 2015;11:1173–5.
Patel D et al. Fel d 1-derived peptide antigen desensitization shows a persistent treatment effect 1 year after the start of dosing: a randomized, placebo-controlled study. J Allergy Clin Immunol. 2013;131:103–9.e1-7.
Couroux P, Patel D, Armstrong K, Larché M, Hafner RP. Fel d 1-derived synthetic peptide immuno-regulatory epitopes show a long-term treatment effect in cat allergic subjects. Clin Exp Allergy. 2015;45:974–81.
Aryan Z, Holgate ST, Radzioch D, Rezaei N. A new era of targeting the ancient gatekeepers of the immune system: toll-like agonists in the treatment of allergic rhinitis and asthma. Int Arch Allergy Immunol. 2014;164:46–63.
Klimek L, Bachmann MF, Senti G, Kündig TM. Immunotherapy of type-1 allergies with virus-like particles and CpG-motifs. Expert Rev Clin Immunol. 2014;10:1059–67.
Kündig TM et al. Is the allergen really needed in allergy immunotherapy? Curr Treat Options Allergy. 2015;2(1):72–82.
Casale TB, Stokes JR. Immunotherapy: what lies beyond. J Allergy Clin Immunol. 2014;133:612–19. This article is a comprehensive review on immunotherapy, covering current indications and future perspective.
Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103:147–66.
Shiraishi Y, Takeda K, Domenico J, Gelfand EW. Role of prostaglandin D2 and CRTH2 blockade in early- and late-phase nasal responses. Clin Exp Allergy. 2014;44:1076–82.
Takahashi G et al. Effect of the potent and selective DP1 receptor antagonist, asapiprant (S-555739), in animal models of allergic rhinitis and allergic asthma. Eur J Pharmacol. 2015;765:15–23.
Nakano Y et al. Role of Prostaglandin D2 and DP1 Receptor on Japanese Cedar Pollen-Induced Allergic Rhinitis in Mice. J Pharmacol Exp Ther. 2016;357:258–63.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Amelia Licari declares that she has no conflict of interest. Gianluigi Marseglia declares that he has no conflict of interest. Giorgio Ciprandi declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Allergic Rhinitis
Rights and permissions
About this article
Cite this article
Licari, A., Marseglia, G. & Ciprandi, G. New Pharmacologic Strategies for Allergic Rhinitis. Curr Treat Options Allergy 3, 495–505 (2016). https://doi.org/10.1007/s40521-016-0105-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40521-016-0105-1