Opinion statement
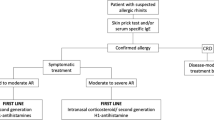
Allergic rhinitis (AR) is one of the most common allergic diseases. Globally, it is estimated to affect more than 500 million people. The burden of allergic rhinitis is overwhelming due to costs caused by sickness absence, medication, and suboptimal performance by the affected population. AR is characterized by a type I allergic reaction in the epithelium of the nose including both an acute phase reaction and a late phase reaction. The allergic reaction causes exudation, itching, sneezing, and later blocking of the nose. Very often, the nasal symptoms are accompanied by eye symptoms (itching and hyperaemia) and oral allergy syndrome. The treatment of AR include patient education, allergen avoidance, pharmacotherapy, and in a selection of patients, allergen immunotherapy (AIT). The cornerstone of pharmacotherapy is the intranasal corticosteroids (INS). Intranasal application of corticosteroids is regarded as a very efficient treatment—treating not only the acute phase symptoms but also the late phase reaction. The treatment is safe and causes only a few well-known side effects. The aim of this review is to provide an overview of all aspects of corticosteroid treatment including mode of application (per oral, intranasal, intramuscular), way of action, potency, bioavailability, side effects, and aspects regarding the pediatric population.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bousquet J, Van Cauwenberge P, Khaltaev N, Aria Workshop G, World Health O. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108:S147–334.
Seidman MD, Gurgel RK, Lin SY, Schwartz SR, Baroody FM, Bonner JR, et al. Clinical practice guideline: allergic rhinitis. Otolaryngol Head Neck Surg. 2015;152:S1–43.
Fardet L, Feve B. Systemic glucocorticoid therapy: a review of its metabolic and cardiovascular adverse events. Drugs. 2014;74:1731–45. This review finds a high incidence of side effects in patients treated with systemic steroids.
Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, Casale TB, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466–76.
Roberts G, Xatzipsalti M, Borrego LM, Custovic A, Halken S, Hellings PW, et al. Paediatric rhinitis: position paper of the European Academy of Allergy and Clinical Immunology. Allergy. 2013;68:1102–16.
Derendorf H, Meltzer EO. Molecular and clinical pharmacology of intranasal corticosteroids: clinical and therapeutic implications. Allergy. 2008;63:1292–300.
Burks AW, Calderon MA, Casale T, Cox L, Demoly P, Jutel M, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;1288:1288–96. This consensus report thoroughly reviews the AIT as a treatment option for AR and asthma.
Radulovic S, Calderon MA, Wilson D, Durham S: Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev 2010:CD002893.
Calderon MA, Alves B, Jacobson M, Hurwitz B, Sheikh A, Durham S: Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev 2007:CD001936.
Vashisht P, Casale T. Omalizumab for treatment of allergic rhinitis. Expert Opin Biol Ther. 2013;13:933–45. This review finds Omalizumab promising as a treatment for otherwise treatment resistant AR.
Ratner PH, Meltzer EO, Teper A. Mometasone furoate nasal spray is safe and effective for 1-year treatment of children with perennial allergic rhinitis. Int J Pediatr Otorhinolaryngol. 2009;73:651–7.
Schenkel EJ, Skoner DP, Bronsky EA, Miller SD, Pearlman DS, Rooklin A, et al. Absence of growth retardation in children with perennial allergic rhinitis after one year of treatment with mometasone furoate aqueous nasal spray. Pediatrics. 2000;105, E22.
Allen DB, Meltzer EO, Lemanske Jr RF, Philpot EE, Faris MA, Kral KM, et al. No growth suppression in children treated with the maximum recommended dose of fluticasone propionate aqueous nasal spray for one year. Allergy Asthma Proc. 2002;23:407–13.
Murphy K, Uryniak T, Simpson B, O'Dowd L. Growth velocity in children with perennial allergic rhinitis treated with budesonide aqueous nasal spray. Ann Allergy Asthma Immunol. 2006;96:723–30.
Lee LA, Sterling R, Maspero J, Clements D, Ellsworth A, Pedersen S. Growth velocity reduced with once-daily fluticasone furoate nasal spray in prepubescent children with perennial allergic rhinitis. J Allergy Clin Immunol Pract. 2014;2:421–7. Large study investigating growth during a one year treatment period with fluticasone furoate finds a small but significant decrease in growth of 0.27 cm.
Skoner DP, Berger WE, Gawchik SM, Akbary A, Qiu C. Intranasal triamcinolone and growth velocity. Pediatrics. 2015;135:e348–356. Large study investigating growth during a one year treatment period with triamcinolone finds a small but significant decrease in growth of 0.45 cm.
Onrust SV, Lamb HM. Mometasone furoate. A review of its intranasal use in allergic rhinitis. Drugs. 1998;56:725–45.
Mygind N, Dahl R. The rationale for use of topical corticosteroids in allergic rhinitis. Clin Exp Allergy. 1996;26 Suppl 3:2–10.
Smith CL, Kreutner W. In vitro glucocorticoid receptor binding and transcriptional activation by topically active glucocorticoids. Arzneimittelforschung. 1998;48:956–60.
Bousquet J, Reid J, van Weel C, Baena Cagnani C, Canonica GW, Demoly P, et al. Allergic rhinitis management pocket reference 2008. Allergy. 2008;63:990–6.
Scadding GK, Durham SR, Mirakian R, Jones NS, Leech SC, Farooque S, et al. BSACI guidelines for the management of allergic and non-allergic rhinitis. Clin Exp Allergy. 2008;38:19–42.
Benninger M, Farrar JR, Blaiss M, Chipps B, Ferguson B, Krouse J, et al. Evaluating approved medications to treat allergic rhinitis in the United States: an evidence-based review of efficacy for nasal symptoms by class. Ann Allergy Asthma Immunol. 2010;104:13–29.
Valotis A, Hogger P. Human receptor kinetics and lung tissue retention of the enhanced-affinity glucocorticoid fluticasone furoate. Respir Res. 2007;8:54.
Corren J. Intranasal corticosteroids for allergic rhinitis: how do different agents compare? J Allergy Clin Immunol. 1999;104:S144–149.
Demoly P. Safety of intranasal corticosteroids in acute rhinosinusitis. Am J Otolaryngol. 2008;29:403–13.
Bende M, Carrillo T, Vona I, da Castel-Branco MG, Arheden L. A randomized comparison of the effects of budesonide and mometasone furoate aqueous nasal sprays on nasal peak flow rate and symptoms in perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2002;88:617–23.
Meltzer EO, Andrews C, Journeay GE, Lim J, Prillaman BA, Garris C, et al. Comparison of patient preference for sensory attributes of fluticasone furoate or fluticasone propionate in adults with seasonal allergic rhinitis: a randomized, placebo-controlled, double-blind study. Ann Allergy Asthma Immunol. 2010;104:331–8.
Khan MA, Abou-Halawa AS, Al-Robaee AA, Alzolibani AA, Al-Shobaili HA. Daily versus self-adjusted dosing of topical mometasone furoate nasal spray in patients with allergic rhinitis: randomised, controlled trial. J Laryngol Otol. 2010;124:397–401.
Kirtsreesakul V, Chansaksung P, Ruttanaphol S. Dose-related effect of intranasal corticosteroids on treatment outcome of persistent allergic rhinitis. Otolaryngol Head Neck Surg. 2008;139:565–9.
Aasbjerg K, Torp-Pedersen C, Vaag A, Backer V. Treating allergic rhinitis with depot-steroid injections increase risk of osteoporosis and diabetes. Respir Med. 2013;107:1852–8. The study finds an increased risk of diabetes and osteoporosis after at least one depot-steroid injection for three consecutive years.
Mygind N, Laursen LC, Dahl M. Systemic corticosteroid treatment for seasonal allergic rhinitis: a common but poorly documented therapy. Allergy. 2000;55:11–5.
Karaki M, Akiyama K, Mori N. Efficacy of intranasal steroid spray (mometasone furoate) on treatment of patients with seasonal allergic rhinitis: comparison with oral corticosteroids. Auris Nasus Larynx. 2013;40:277–81.
Meltzer EO, Tripathy I, Maspero JF, Wu W, Philpot E. Safety and tolerability of fluticasone furoate nasal spray once daily in paediatric patients aged 6–11 years with allergic rhinitis: subanalysis of three randomized, double-blind, placebo-controlled, multicentre studies. Clin Drug Investig. 2009;29:79–86.
Kumar R, Kumar D, Parakh A. Fluticasone furoate: a new intranasal corticosteroid. J Postgrad Med. 2012;58:79–83.
Patel D, Ratner P, Clements D, Wu W, Faris M, Philpot E. Lack of effect on adult and adolescent hypothalamic-pituitary-adrenal axis function with use of fluticasone furoate nasal spray. Ann Allergy Asthma Immunol. 2008;100:490–6.
Bruni FM, De Luca G, Venturoli V, Boner AL. Intranasal corticosteroids and adrenal suppression. Neuroimmunomodulation. 2009;16:353–62.
Skoner DP, Rachelefsky GS, Meltzer EO, Chervinsky P, Morris RM, Seltzer JM, et al. Detection of growth suppression in children during treatment with intranasal beclomethasone dipropionate. Pediatrics. 2000;105, E23.
Derby L, Maier WC. Risk of cataract among users of intranasal corticosteroids. J Allergy Clin Immunol. 2000;105:912–6.
Lightman S, Scadding GK. Should intranasal corticosteroids be used for the treatment of ocular symptoms of allergic rhinoconjunctivitis? A review of their efficacy and safety profile. Int Arch Allergy Immunol. 2012;158:317–25. This review does not find a higher frequency of glaucoma and cataracts in the INS users compared to non-users.
Hedner P, Persson G. Suppression of the hypothalamic-pituitary-adrenal axis after a single intramuscular injection of methylprednisolone acetate. Ann Allergy. 1981;47:176–9.
Ameratunga R. Gluteal subcutaneous atrophy after depot steroid injection for allergic rhinitis. World Allergy Organ J. 2012;5:168–9.
Fernandes RM, Oleszczuk M, Woods CR, Rowe BH, Cates CJ, Hartling L. The Cochrane library and safety of systemic corticosteroids for acute respiratory conditions in children: an overview of reviews. Evid Based Child Health. 2014;9:733–47. The review finds it safe to use oral steroids as a short term treatment in children.
Katelaris CH, Sacks R, Theron PN. Allergic rhinoconjunctivitis in the Australian population: burden of disease and attitudes to intranasal corticosteroid treatment. Am J Rhinol Allergy. 2013;27:506–9.
Lin SY, Melvin TA, Boss EF, Ishman SL. The association between allergic rhinitis and sleep-disordered breathing in children: a systematic review. Int Forum Allergy Rhinol. 2013;3:504–9.
Lavigne F, Petrof BJ, Johnson JR, Lavigne P, Binothman N, Kassissia GO, et al. Effect of topical corticosteroids on allergic airway inflammation and disease severity in obstructive sleep apnoea. Clin Exp Allergy. 2013;43:1124–33. The study finds a direct causation between sleep-disordered breathing and AR.
Walker S, Khan-Wasti S, Fletcher M, Cullinan P, Harris J, Sheikh A. Seasonal allergic rhinitis is associated with a detrimental effect on examination performance in United Kingdom teenagers: case-control study. J Allergy Clin Immunol. 2007;120:381–7.
Kim DK, Rhee CS, Han DH, Won TB, Kim DY, Kim JW. Treatment of allergic rhinitis is associated with improved attention performance in children: the Allergic Rhinitis Cohort Study for Kids (ARCO-kids). PLoS One. 2014;9, e109145.
Fokkens WJ, Jogi R, Reinartz S, Sidorenko I, Sitkauskiene B, van Oene C, et al. Once daily fluticasone furoate nasal spray is effective in seasonal allergic rhinitis caused by grass pollen. Allergy. 2007;62:1078–84.
Bender BG, Milgrom H. Comparison of the effects of fluticasone propionate aqueous nasal spray and loratadine on daytime alertness and performance in children with seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2004;92:344–9.
Sardana N, Craig TJ. Congestion and sleep impairment in allergic rhinitis. Asian Pac J Allergy Immunol. 2011;29:297–306.
Thompson A, Sardana N, Craig TJ. Sleep impairment and daytime sleepiness in patients with allergic rhinitis: the role of congestion and inflammation. Ann Allergy Asthma Immunol. 2013;111:446–51.
Simons FE, Simons KJ. Histamine and H1-antihistamines: celebrating a century of progress. J Allergy Clin Immunol. 2011;128:1139–50. e1134.
Nasser M, Fedorowicz Z, Aljufairi H, McKerrow W: Antihistamines used in addition to topical nasal steroids for intermittent and persistent allergic rhinitis in children. Cochrane Database Syst Rev 2010:CD006989.
Bousquet J, Bachert C, Bernstein J, Canonica GW, Carr W, Dahl R, et al. Advances in pharmacotherapy for the treatment of allergic rhinitis; MP29-02 (a novel formulation of azelastine hydrochloride and fluticasone propionate in an advanced delivery system) fills the gaps. Expert Opin Pharmacother. 2015;16:913–28. The study describes a novel formulation of the combination of azelastine and fluticasone propionate as fast acting and with a higher grade of relief of all symptoms than the two drugs used separately.
Meltzer E, Ratner P, Bachert C, Carr W, Berger W, Canonica GW, et al. Clinically relevant effect of a new intranasal therapy (MP29-02) in allergic rhinitis assessed by responder analysis. Int Arch Allergy Immunol. 2013;161:369–77.
Carr W, Bernstein J, Lieberman P, Meltzer E, Bachert C, Price D, et al. A novel intranasal therapy of azelastine with fluticasone for the treatment of allergic rhinitis. J Allergy Clin Immunol. 2012;129:1282–9. e1210.
Nayak A, Langdon RB. Montelukast in the treatment of allergic rhinitis: an evidence-based review. Drugs. 2007;67:887–901.
Goh BS, Ismail MI, Husain S. Quality of life assessment in patients with moderate to severe allergic rhinitis treated with montelukast and/or intranasal steroids: a randomised, double-blind, placebo-controlled study. J Laryngol Otol. 2014;128:242–8.
Platt M. Pharmacotherapy for allergic rhinitis. Int Forum Allergy Rhinol. 2014;4 Suppl 2:S35–40.
Baroody FM, Brown D, Gavanescu L, DeTineo M, Naclerio RM. Oxymetazoline adds to the effectiveness of fluticasone furoate in the treatment of perennial allergic rhinitis. J Allergy Clin Immunol. 2011;127:927–34.
Casale TB, Condemi J, LaForce C, Nayak A, Rowe M, Watrous M, et al. Effect of omalizumab on symptoms of seasonal allergic rhinitis: a randomized controlled trial. JAMA. 2001;286:2956–67.
Ogino S, Nagakura T, Okubo K, Sato N, Takahashi M, Ishikawa T. Re-treatment with omalizumab at one year interval for Japanese cedar pollen-induced seasonal allergic rhinitis is effective and well tolerated. Int Arch Allergy Immunol. 2009;149:239–45.
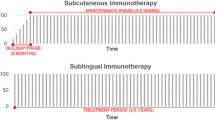
Dretzke J, Meadows A, Novielli N, Huissoon A, Fry-Smith A, Meads C. Subcutaneous and sublingual immunotherapy for seasonal allergic rhinitis: a systematic review and indirect comparison. J Allergy Clin Immunol. 2013;131:1361–6. The review thoroughly compare the treatment with subcutaneous and sublingual immunotherapy and conclude that the treatments are equally effective.
Marogna M, Spadolini I, Massolo A, Canonica GW, Passalacqua G. Long-lasting effects of sublingual immunotherapy according to its duration: a 15-year prospective study. J Allergy Clin Immunol. 2010;126:969–75.
Moller C, Dreborg S, Ferdousi HA, Halken S, Host A, Jacobsen L, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study). J Allergy Clin Immunol. 2002;109:251–6.
Casale TB, Busse WW, Kline JN, Ballas ZK, Moss MH, Townley RG, et al. Omalizumab pretreatment decreases acute reactions after rush immunotherapy for ragweed-induced seasonal allergic rhinitis. J Allergy Clin Immunol. 2006;117:134–40.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Agertoft reports personal fees from ALK, personal fees from Novartis, personal fees from Nigaard, and study fees from ALK, outside the submitted work.
Dr. Petersen reports personal fees from Teva Demark A/S and a sponsored study for ALK Abelló.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Allergic Rhinitis
Rights and permissions
About this article
Cite this article
Petersen, T.H., Agertoft, L. Corticosteroids for Allergic Rhinitis. Curr Treat Options Allergy 3, 18–30 (2016). https://doi.org/10.1007/s40521-016-0075-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40521-016-0075-3