Abstract
Background
Osteoporosis is a degenerative disease defined by low bone mineral density, has a high prevalence, and causes fractures at multiple sites throughout the body, greatly affecting the quality of patients. α-Klotho is an endocrine factor involved in the regulation of various metabolic processes in humans, and its role in bone metabolism has attracted widespread attention. The relationship between α-klotho and bone mineral density has not been uniformly recognized, and no large-scale correlation analysis has been conducted in the middle-aged and elderly population.
Objective
To determine the relationship between α-klotho and bone mineral density in middle-aged and elderly people.
Methods
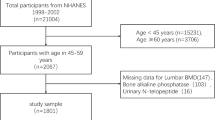
Population data of 3120 individuals aged 40–79 years were obtained from the NHANES database for the period 2011–2016. Regression analysis was performed using a general linear model with serum α-klotho as the independent variable and total bone mineral density, thoracic bone mineral density, lumbar bone mineral density, pelvic bone mineral density, and trunk bone mineral density as the dependent variables, respectively. The generalized additive model was also used for smoothing curve fitting and threshold effect analysis.
Results
Serum α-klotho was positively correlated with total bone mineral density at lg (Klotho) < 2.97 and with thoracic bone mineral density at lg (Klotho) > 2.69 (β = 0.05, p = 0.0006), and negatively correlated (β = −0.27, p = 0.0341) with lumbar bone mineral density at lg (Klotho) < 2.69. It also positively correlated with trunk bone mineral density (β = 0.027, p = 0.03657) and had no segmental effect but did not correlate with pelvic bone mineral density. The positive association of serum α-klotho with those aged 40–49 years, female, non-Hispanic White, and without hypertension was clearer. In the population with diabetes, a significantly positive association between total (β = 0.15, p = 0.01), thoracic (β = 0.23, p = 0.0404), and lumbar (β = 0.22, p = 0.0424) bone mineral density and α-klotho was observed.
Conclusions
α-Klotho has different relationships with total, thoracic, lumbar, and trunk bone mineral density. Among them, the positive correlation between α-klotho and trunk bone mineral density is more valuable for predicting osteoporosis. The significant effect of α-klotho on bone mineral density in diabetes patients suggests its potential as a predictive marker of diabetes progression.


Similar content being viewed by others
Data availability
Publicly available datasets were analyzed in this study. These data can be found here: https://www.cdc.gov/nchs/nhanes/.
References
Klibanski A, Adams-Campbell L, Bassford T et al (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA J am Med Assoc 285:785–795
Gargiulo P, Helgason T, Ramon C et al (2014) CT and MRI assessment and characterization using segmentation and 3D modeling techniques: applications to muscle, bone and brain. Eur J Transl Myol 24:3298. https://doi.org/10.4081/ejtm.2014.3298
Recenti M, Ricciardi C, Edmunds K et al (2020) Machine learning predictive system based upon radiodensitometric distributions from mid-thigh CT images. Eur J Transl Myol 30:8892. https://doi.org/10.4081/ejtm.2019.8892
Ciliberti FK, Cesarelli G, Guerrini L et al (2022) The role of bone mineral density and cartilage volume to predict knee cartilage degeneration. Eur J Transl Myol 32:10678. https://doi.org/10.4081/ejtm.2022.10678
Kanis JA, McCloskey EV, Johansson H et al (2008) A reference standard for the description of osteoporosis. Bone 42:467–475. https://doi.org/10.1016/j.bone.2007.11.001
Kanis JA (2002) Diagnosis of osteoporosis and assessment of fracture risk. Lancet 359:1929–1936. https://doi.org/10.1016/s0140-6736(02)08761-5
Ensrud KE, Kats AM, Boyd CM et al (2019) Association of disease definition, comorbidity burden, and prognosis with hip fracture probability among late-life women. JAMA Intern Med 179:1095–1103. https://doi.org/10.1001/jamainternmed.2019.0682
Stanghelle B, Bentzen H, Giangregorio L et al (2019) Associations between health-related quality of life, physical function and pain in older women with osteoporosis and vertebral fracture. BMC Geriatr 19:298. https://doi.org/10.1186/s12877-019-1268-y
Nawrat-Szoltysik A, Miodonska Z, Piejko L et al (2021) Assessment of quality of life and pain severity in older men with osteoporosis: cross-sectional study. Int J Env Res Pub He 18:11276. https://doi.org/10.3390/ijerph182111276
Frost M, Wraae K, Abrahamsen B et al (2012) Osteoporosis and vertebral fractures in men aged 60–74 years. Age Ageing 41:171–177. https://doi.org/10.1093/ageing/afr170
Kuro-o M, Matsumura Y, Aizawa H et al (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390:45–51. https://doi.org/10.1038/36285
Olauson H, Mencke R, Hillebrands J-L et al (2017) Tissue expression and source of circulating alpha tKlotho. Bone 100:19–35. https://doi.org/10.1016/j.bone.2017.03.043
Lindberg K, Amin R, Moe OW et al (2014) The kidney is the principal organ mediating klotho effects. J Am Soc Nephrol 25:2169–2175. https://doi.org/10.1681/asn.2013111209
Xu Y, Sun Z (2015) Molecular basis of Klotho: from gene to function in aging. Endocr Rev 36:174–193. https://doi.org/10.1210/er.2013-1079
Ohsawa Y, Ohtsubo H, Munekane A et al (2023) Circulating α-klotho counteracts transforming growth factor-β-induced sarcopenia. Am J Pathol. https://doi.org/10.1016/j.ajpath.2023.01.009
Nakao VW, Mazucanti CHY, de Sá LL et al (2022) Neuroprotective action of α-Klotho against LPS-activated glia conditioned medium in primary neuronal culture. Sci Rep 12:18884. https://doi.org/10.1038/s41598-022-21132-4
Komaba H, Kaludjerovic J, Hu DZ et al (2017) Klotho expression in osteocytes regulates bone metabolism and controls bone formation. Kidney Int 92:599–611. https://doi.org/10.1016/j.kint.2017.02.014
Hamdy M, Shaheen I, Seif El Din H et al (2022) Klotho level as a marker of low bone mineral density in Egyptian sickle cell disease patients. J Pediatr Hematol Oncol 44:e40–e45. https://doi.org/10.1097/mph.0000000000002231
Zheng S, Chen Y, Zheng Y et al (2018) Correlation of serum levels of fibroblast growth factor 23 and klotho protein levels with bone mineral density in maintenance hemodialysis patients. Eur J Med Res 23:18. https://doi.org/10.1186/s40001-018-0315-z
Matei A, Bilha SC, Constantinescu D et al (2022) Body composition, adipokines, FGF23-klotho and bone in kidney transplantation: is there a link? J Nephrol 35:293–304. https://doi.org/10.1007/s40620-021-00972-9
Amaro-Gahete FJ, De-la OA, Jurado-Fasoli L et al (2019) Body composition and S-klotho plasma levels in middle-aged adults: a cross-sectional study. Rejuvenat Res 22:478–483. https://doi.org/10.1089/rej.2018.2092
Chen TC, Clark J, Riddles MK et al (2020) National Health and Nutrition Examination Survey, 2015–2018: Sample Design and Estimation Procedures. Vital Health Stat 2:1–35
Alaimo K, Briefel RR, Frongillo EA Jr et al (1998) Food insufficiency exists in the United States: results from the third National Health and Nutrition Examination Survey (NHANES III). Am J Public Health 88:419–426. https://doi.org/10.2105/ajph.88.3.419
Sjostrom M, Ainsworth BE, Bauman A et al (2005) Guidelines for data processing analysis of the International Physical Activity Questionnaire (IPAQ)—short and long forms
Flegal KM, Kruszon-Moran D, Carroll MD et al (2016) Trends in obesity among adults in the United States, 2005 to 2014. JAMA 315:2284–2291. https://doi.org/10.1001/jama.2016.6458
Chen M, Yang Y, Baral K et al (2023) Relationship between bisphenol A and the cardiovascular disease metabolic risk factors in American adults: a population-based study. Chemosphere 324: 138289. https://doi.org/10.1016/j.chemosphere.2023.138289
Kim D, Lee S, Choi JY et al (2022) Association of α-klotho and lead and cadmium: a cross-sectional study. Sci Total Environ 843: 156938. https://doi.org/10.1016/j.scitotenv.2022.156938
Pedersen L, Pedersen SM, Brasen CL et al (2013) Soluble serum klotho levels in healthy subjects. Comparison of two different immunoassays. Clin Biochem 46:1079–1083. https://doi.org/10.1016/j.clinbiochem.2013.05.046
Kurosu H, Ogawa Y, Miyoshi M et al (2006) Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 281:6120–6123. https://doi.org/10.1074/jbc.C500457200
Goetz R, Nakada Y, Hu MC et al (2010) Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23–FGFR–klotho complex formation. Proc Natl Acad Sci USA 107:407–412. https://doi.org/10.1073/pnas.0902006107
Urakawa I, Yamazaki Y, Shimada T et al (2006) Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444:770–774. https://doi.org/10.1038/nature05315
Andrukhova O, Smorodchenko A, Egerbacher M et al (2014) FGF23 promotes renal calcium reabsorption through the TRPV5 channel. Embo J 33:229–246. https://doi.org/10.1002/embj.201284188
Zhu D, Mackenzie NC, Millan JL et al (2013) A protective role for FGF-23 in local defence against disrupted arterial wall integrity? Mol Cell Endocrinol 372:1–11. https://doi.org/10.1016/j.mce.2013.03.008
Hu MC, Shi M, Zhang J et al (2011) Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 22:124–136. https://doi.org/10.1681/ASN.2009121311
Ribeiro AL, Mendes F, Carias E et al (2020) FGF23-klotho axis as predictive factors of fractures in type 2 diabetics with early chronic kidney disease. J Diabetes Complicat 34: 107476. https://doi.org/10.1016/j.jdiacomp.2019.107476
Kawano K, Ogata N, Chiano M et al (2002) Klotho gene polymorphisms associated with bone density of aged postmenopausal women. J Bone Miner Res 17:1744–1751. https://doi.org/10.1359/jbmr.2002.17.10.1744
Mullin BH, Wilson SG, Islam FM et al (2005) Klotho gene polymorphisms are associated with osteocalcin levels but not bone density of aged postmenopausal women. Calcif Tissue Int 77:145–151. https://doi.org/10.1007/s00223-004-0291-x
Yuan Q, Sato T, Densmore M et al (2012) Deletion of PTH rescues skeletal abnormalities and high osteopontin levels in Klotho−/− mice. PLoS Genet 8: e1002726. https://doi.org/10.1371/journal.pgen.1002726
Neyra JA, Hu MC (2016) αKlotho and chronic kidney disease. Vitam Horm 101:257–310. https://doi.org/10.1016/bs.vh.2016.02.007
Razzaque MS (2012) FGF23, klotho and vitamin D interactions: what have we learned from in vivo mouse genetics studies? Adv Exp Med Biol 728:84–91. https://doi.org/10.1007/978-1-4614-0887-1_5
Wolf I, Stein D, Shahmoon S et al (2016) Alteration in serum klotho levels in anorexia nervosa patients. Clin Nutr 35:958–962. https://doi.org/10.1016/j.clnu.2015.07.013
Marchelek-Myśliwiec M, Dziedziejko V, Nowosiad-Magda M et al (2019) Bone metabolism parameters in hemodialysis patients with chronic kidney disease and in patients after kidney transplantation. Physiol Res 68: 947–954. https://doi.org/10.33549/physiolres.934118
Wang K, Mao Y, Lu M et al (2022) Association between serum klotho levels and the prevalence of diabetes among adults in the United States. Front Endocrinol (Lausanne) 13:1005553. https://doi.org/10.3389/fendo.2022.1005553
Devaraj S, Syed B, Chien A et al (2012) Validation of an immunoassay for soluble klotho protein decreased levels in diabetes and increased levels in chronic kidney disease. Am J Clin Pathol 137:479–485. https://doi.org/10.1309/AJCPGPMAF7SFRBO4
Nie F, Wu DM, Du HF et al (2017) Serum klotho protein levels and their correlations with the progression of type 2 diabetes mellitus. J Diabetes Complicat 31:594–598. https://doi.org/10.1016/j.jdiacomp.2016.11.008
Hu MC, Shi M, Zhang J et al (2016) Renal production, uptake, and handling of circulating αklotho. J Am Soc Nephrol 27:79–90. https://doi.org/10.1681/asn.2014101030
Kurra S, Siris E (2011) Diabetes and bone health: the relationship between diabetes and osteoporosis-associated fractures. Diabetes Metab Res Rev 27:430–435. https://doi.org/10.1002/dmrr.1197
Leidig-Bruckner G, Grobholz S, Bruckner T et al (2014) Prevalence and determinants of osteoporosis in patients with type 1 and type 2 diabetes mellitus. BMC Endocr Disord 14:33. https://doi.org/10.1186/1472-6823-14-33
Acknowledgements
The authors thank all of the participants, coordinators, and administrators for their support and help during the research.
Funding
This study was supported by the National Natural Science Foundation of China (82270795, 81700658), the Hunan Provincial Natural Science Foundation (2020JJ3058) and the Wisdom Accumulation and Talent Cultivation Project of the Third Xiangya Hospital of Central South University (YX202212).
Author information
Authors and Affiliations
Contributions
Yang Zhang: conceptualization, methodology, formal analysis, investigation, data curation, writing—review and editing, visualization. Changtai Zhao: conceptualization, methodology, formal analysis, data curation, visualization, writing—original draft. Hanyong Zhang: conceptualization, formal analysis, investigation, supervision, writing—review and editing. Mongcong Chen: conceptualization, formal analysis, investigation, writing—review and editing. Yang Meng: conceptualization, data curation, formal analysis, investigation, methodology. Yuxin Pan: conceptualization, data curation, formal analysis, visualization, writing—review and editing. Quan Zhuang: conceptualization, methodology, funding acquisition, formal analysis, project administration, writing—review and editing, supervision. Mingyi Zhao: conceptualization, methodology, funding acquisition, project administration, writing—review and editing, supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this study.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the National Center for Health Statistics (NCHS) Research Ethics Review Board.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Zhao, C., Zhang, H. et al. Association between serum soluble α-klotho and bone mineral density (BMD) in middle-aged and older adults in the United States: a population-based cross-sectional study. Aging Clin Exp Res 35, 2039–2049 (2023). https://doi.org/10.1007/s40520-023-02483-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-023-02483-y