Abstract
Background
Body composition strongly influences physical function in older adults. Bioelectrical impedance analysis (BIA) differentiates fat mass from skeletal muscle mass, and may be more useful than body mass index (BMI) for classifying women on their likelihood of physical function impairment.
Aims
This study tested whether BIA-derived estimates of percentage body fat (%BF) and height-normalized skeletal muscle mass (skeletal muscle mass index; SMI) enhance classification of physical function impairment relative to BMI.
Method
Black, White, Chinese, and Japanese midlife women (N = 1482) in the Study of Women’s Health Across the Nation (SWAN) completed performance-based measures of physical function. BMI (kg/m2) was calculated. %BF and SMI were derived through BIA. Receiver-operating characteristic (ROC) curve analysis, conducted in the overall sample and stratified by racial group, evaluated optimal cutpoints of BMI, %BF, and SMI for classifying women on moderate–severe physical function impairment.
Results
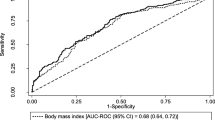
In the overall sample, a BMI cutpoint of ≥ 30.1 kg/m2 correctly classified 71.1% of women on physical function impairment, and optimal cutpoints for %BF (≥ 43.4%) and SMI (≥ 8.1 kg/m2) correctly classified 69% and 62% of women, respectively. SMI did not meaningfully enhanced classification relative to BMI (change in area under the ROC curve = 0.002; net reclassification improvement = 0.021; integrated discrimination improvement = − 0.003). Optimal cutpoints for BMI, %BF, and SMI varied substantially across race. Among Black women, a %BF cutpoint of 43.9% performed somewhat better than BMI (change in area under the ROC curve = 0.017; sensitivity = 0.69, specificity = 0.64).
Conclusion
Some race-specific BMI and %BF cutpoints have moderate utility for identifying impaired physical function among midlife women.

Similar content being viewed by others
References
Metti AL, Best JR, Shaaban CE et al (2018) Longitudinal changes in physical function and physical activity in older adults. Age Ageing 47:558–564. https://doi.org/10.1093/ageing/afy025
Windham BG, Griswold ME, Wang W et al (2017) The importance of mid-to-late-life body mass index trajectories on late-life gait speed. J Gerontol Ser A Biol Sci Med Sci 72:1130–1136. https://doi.org/10.1093/gerona/glw200
Ylitalo KR, Karvonen-Gutierrez CA, Fitzgerald N et al (2013) Relationship of race-ethnicity, body mass index, and economic strain with longitudinal self-report of physical functioning: the Study of Women’s Health Across the Nation. Ann Epidemiol 23:401–408. https://doi.org/10.1016/j.annepidem.2013.04.008
Bea JW, Going SB, Wertheim BC et al (2018) Body composition and physical function in the women’s health initiative observational study. Prev Med Rep 11:15–22. https://doi.org/10.1016/j.pmedr.2018.05.007
Kim S, Leng XI, Kritchevsky SB (2017) Body composition and physical function in older adults with various comorbidities. Innov Aging 1:igx008. https://doi.org/10.1093/geroni/igx008
Reinders I, Murphy RA, Martin KR et al (2015) Body mass index trajectories in relation to change in lean mass and physical function: the health, aging and body composition study. J Am Geriatr Soc 63:1615–1621. https://doi.org/10.1111/jgs.13524
Mongraw-Chaffin M, Golden SH, Allison MA et al (2015) The sex and race specific relationship between anthropometry and body fat composition determined from computed tomography: evidence from the multi-ethnic study of atherosclerosis. PLoS One 10:e0139559. https://doi.org/10.1371/journal.pone.0139559
Yilmaz O, Bahat G (2017) Suggestions for assessment of muscle mass in primary care setting. Aging Male 20:168–169. https://doi.org/10.1080/13685538.2017.1311856
Wheaton FV, Crimmins EM (2016) Female disability disadvantage: a global perspective on sex differences in physical function and disability. Aging Soc 36:1136–1156. https://doi.org/10.1017/s0144686x15000227
Greendale GA, Sternfeld B, Huang M et al (2019) Changes in body composition and weight during the menopause transition. JCI Insight 4:124865. https://doi.org/10.1172/jci.insight.124865
Sowers MR, Crawford SL, Sternfeld B et al (2000) SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey JL, Marcus R (eds) Menopause: biology and pathobiology. Academic Press, San Diego, pp 175–188
Guralnik JM, Simonsick EM, Ferrucci L et al (1994) A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 49:M85–M94
Miller DK, Wolinsky FD, Andresen EM et al (2008) Adverse outcomes and correlates of change in the short physical performance battery over 36 months in the African American health project. J Gerontol Ser A Biol Sci Med Sci 63:487–494
Chumlea WC, Guo SS, Kuczmarski RJ et al (2002) Body composition estimates from NHANES III bioelectrical impedance data. Int J Obes 26:1596–1609. https://doi.org/10.1038/sj.ijo.0802167
Janssen I, Heymsfield SB, Baumgartner RN et al (2000) Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol 89:465–471. https://doi.org/10.1152/jappl.2000.89.2.465
Janssen I, Baumgartner RN, Ross R et al (2004) Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol 159:413–421
Perkins NJ, Schisterman EF (2005) The Youden index and the optimal cut-point corrected for measurement error. Biom J 47:428–441
Pencina MJ, D’Agostino RB, Pencina KM et al (2012) Interpreting incremental value of markers added to risk prediction models. Am J Epidemiol 176:473–481. https://doi.org/10.1093/aje/kws207
Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr et al (2008) Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 27:157–172. https://doi.org/10.1002/sim.2929
Pencina MJ, D’Agostino RB Sr, Steyerberg EW (2011) Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med 30:11–21. https://doi.org/10.1002/sim.4085
Pencina MJ, Fine JP, D’Agostino RB (2017) Discrimination slope and integrated discrimination improvement—properties, relationships and impact of calibration. Stat Med 36:4482–4490. https://doi.org/10.1002/sim.7139
Kerr KF, McClelland RL, Brown ER et al (2011) Evaluating the incremental value of new biomarkers with integrated discrimination improvement. Am J Epidemiol 174:364–374. https://doi.org/10.1093/aje/kwr086
World Health Organization (2000) Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organization technical report series 894:1–253
Ward CL, Valentine RJ, Evans EM (2014) Greater effect of adiposity than physical activity or lean mass on physical function in community-dwelling older adults. J Aging Phys Act 22:284–293. https://doi.org/10.1123/japa.2012-0098
Woo J, Leung J, Kwok T (2007) BMI, body composition, and physical functioning in older adults. Obesity 15:1886–1894. https://doi.org/10.1038/oby.2007.223
Sternfeld B, Ngo L, Satariano WA et al (2002) Associations of body composition with physical performance and self-reported functional limitation in elderly men and women. Am J Epidemiol 156:110–121
Kim YH, Kim KI, Paik NJ et al (2016) Muscle strength: a better index of low physical performance than muscle mass in older adults. Geriatr Gerontol Int 16:577–585. https://doi.org/10.1111/ggi.12514
Therkelsen KE, Pedley A, Hoffmann U et al (2016) Intramuscular fat and physical performance at the Framingham heart study. Age 38:31. https://doi.org/10.1007/s11357-016-9893-2
Gonzalez MC, Correia M, Heymsfield SB (2017) A requiem for BMI in the clinical setting. Curr Opin Clin Nutr Metab Care 20:314–321. https://doi.org/10.1097/mco.0000000000000395
Muscaritoli M, Anker SD, Argiles J et al (2010) Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by special interest groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr 29:154–159. https://doi.org/10.1016/j.clnu.2009.12.004
Cruz-Jentoft AJ, Baeyens JP, Bauer JM et al (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Aging 39:412–423. https://doi.org/10.1093/ageing/afq034
Fielding RA, Vellas B, Evans WJ et al (2011) Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 12:249–256. https://doi.org/10.1016/j.jamda.2011.01.003
Batsis JA, Mackenzie TA, Lopez-Jimenez F et al (2015) Sarcopenia, sarcopenic obesity, and functional impairments in older adults: national health and nutrition examination surveys 1999–2004. Nutr Res 35:1031–1039. https://doi.org/10.1016/j.nutres.2015.09.003
Houston DK, Ding J, Nicklas BJ et al (2005) The association between weight history and physical performance in the health, aging and body composition study. Int J Obes 31:1680–1687. https://doi.org/10.1038/sj.ijo.0803652
Santanasto AJ, Glynn NW, Lovato LC et al (2017) Effect of physical activity versus health education on physical function, grip strength and mobility. J Am Geriatr Soc 65:1427–1433. https://doi.org/10.1111/jgs.14804
Sims ST, Kubo J, Desai M et al (2013) Changes in physical activity and body composition in postmenopausal women over time. Med Sci Sports Exerc 45:1486–1492. https://doi.org/10.1249/MSS.0b013e31828af8bd
Acknowledgements
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR), and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or the NIH.
Clinical centers: University of Michigan, Ann Arbor—Siobán Harlow, PI 2011–present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA—Joel Finkelstein, PI 1999–present; Robert Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, IL—Howard Kravitz, PI 2009–present; Lynda Powell, PI 1994–2009; University of California, Davis/Kaiser—Ellen Gold, PI; University of California, Los Angeles—Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY—Carol Derby, PI 2011–present, Rachel Wildman, PI 2010–2011; Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry–New Jersey Medical School, Newark—Gerson Weiss, PI 1994–2004; and the University of Pittsburgh, Pittsburgh, PA: Karen Matthews, PI.
NIH program office: National Institute on Aging, Bethesda, MD—Chhanda Dutta 2016–present; Winifred Rossi 2012–2016; Sherry Sherman 1994–2012; Marcia Ory 1994–2001; National Institute of Nursing Research, Bethesda, MD—Program Officers.
Central laboratory: University of Michigan, Ann Arbor—Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating center: University of Pittsburgh, Pittsburgh, PA—Maria Mori Brooks, PI 2012–present; Kim Sutton-Tyrrell, PI 2001–2012; New England Research Institutes, Watertown, MA—Sonja McKinlay, PI 1995–2001.
Steering committee: Susan Johnson, Current Chair. Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of human and animal rights
Study procedures received institutional review board approval from each study site. The study was conducted in accordance with the Declaration of Helsinki.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
40520_2019_1355_MOESM1_ESM.tif
Supplementary material 1. Supplemental Fig. 1. Race-specific receiver-operating characteristic curves for each body composition measure in classifying midlife women on their likelihood of moderate–severe physical function impairment (TIFF 1275 kb)
Rights and permissions
About this article
Cite this article
Appelhans, B.M., Lange-Maia, B.S., Pettee Gabriel, K. et al. Body mass index versus bioelectrical impedance analysis for classifying physical function impairment in a racially diverse cohort of midlife women: the Study of Women’s Health Across the Nation (SWAN). Aging Clin Exp Res 32, 1739–1747 (2020). https://doi.org/10.1007/s40520-019-01355-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-019-01355-8