Abstract
Endurance and strength training are effective strategies for counteracting age-associated reductions in physical performance in older adults, with a combination of both exercise modes recommended to maximise potential fitness benefits. This meta-analysis sought to quantify the effects of same-session combined endurance and strength training on fitness in adults aged over 50 years. Five electronic databases were searched with studies required to include one of the following outcome measures: VO2peak, 6-min walk test (6MWT), 8-ft timed up-and-go (TUG), and 30-s chair stand. Separate random-effects meta-analyses compared combined training with (1) no-exercise control, (2) endurance training, and (3) strength training with probabilistic magnitude-based inferences subsequently applied. Twenty-seven studies involving 1346 subjects with a mean age of 68.8 years (range 54–85 years) were included in the analysis. The meta-analysed effect on VO2peak was a moderately beneficial effect for the combined training compared to no-exercise controls (3.6 mL kg−1 min−1; ± 95% confidence limits 0.8 mL kg−1 min−1) with additional increases for studies with greater proportions of female participants and shorter training interventions. Combined training also had small-to-moderately beneficial effects on VO2peak when compared to endurance training (0.8 mL kg−1 min−1; ± 1.0 mL kg−1 min−1), 30-s chair stand when compared with strength training (1.1 repetitions; ± 0.5 repetitions) and on TUG (0.8 s; ± 0.7 s), 30-s chair stand (2.8 repetitions; ± 1.7 repetitions), and 6MWT (31.5 m; ± 22.4 m) when compared to no-exercise controls. All other comparisons were unclear. Same-session combined training can induce clinically relevant fitness improvements in older adults.
Similar content being viewed by others
Introduction
Human ageing is associated with progressive declines across multiple physiological systems, with changes in the cardiorespiratory and neuromuscular systems some of the most pronounced. Reduced levels of cardiorespiratory [1] and muscular fitness [2] have been associated with increased mortality and morbidity. Improving both of these physical components offers the most effective strategy to reduce all-cause and cardiovascular mortality risk [3]. However, despite the implications of reduced physiological functioning on disease risk and lifespan, maintaining an independent and inclusive lifestyle may be of greater relevance to older adults. Previous work has demonstrated that cardiorespiratory fitness is related to functional capacity and the odds of independent living [4, 5], while muscular fitness (e.g., muscular strength and power) is a critical determinant of physical functioning in older adults [6, 7]. Despite the inevitable declines in physiological functioning observed with ageing, older adults remain highly trainable into advanced age with substantial fitness improvements possible following short-term training programmes [8, 9]. As higher levels of both cardiorespiratory and muscular fitness are related to improved functional performance and reduced mortality risk, these physical components are key targets for intervention.
Traditionally, endurance-type activities (e.g., running, cycling) are prescribed for improving cardiorespiratory fitness with muscle-strengthening activities (e.g., free weights, resistance machines, elastic resistance bands) used to improve muscular fitness. However, as neither endurance nor strength training performed in isolation promotes holistic fitness improvement, exercise recommendations for older adults typically advocate training programmes consisting of a combination of endurance and strength training activities [10, 11]. As a result, ‘combined’ or ‘concurrent’ training programmes—involving endurance and strength training performed within the same, or separate exercise sessions of a training programme, respectively—are commonly prescribed. This approach has been suggested to be a more effective strategy than either endurance or strength training performed alone because of the potential to impact upon multiple components of fitness simultaneously [10, 12]. Recent observational data support this assertion as older adults who meet both the endurance and muscle-strengthening activity guidelines perform significantly better on measures of muscular and functional fitness [13].
However, despite the well-documented and wide-ranging benefits of exercise training, the requirement to perform separate endurance and strength training sessions places considerable time demands on individuals. This remains an important consideration in a population where adherence to exercise guidelines remains poor [14] and where lack of time remains one of the most commonly cited barriers to exercise [15]. Consequently, training programmes involving a reduced time commitment via delivery of endurance and strength training within the same training session may be a more time-efficient, and thereby, attractive proposition for potential exercisers. It seems feasible to suggest that asking individuals to complete a reduced frequency of exercise sessions per week may be more achievable.
Although previous work has reviewed strategies and provided recommendations for the prescription of combined exercise training in older adults [12], there is currently no systematic review examining the effect of same-session combined exercise training on measures of fitness in older adults. Accordingly, the aim of our investigation was to systematically review and meta-analyse the effects of same-session combined exercise training on measures of fitness in adults aged over 50 years, while also exploring the modifying effects of study and subject characteristics. By doing so, we aimed to provide a practically relevant quantification of this training approach to support clinicians and practitioners to make informed decisions relating to the prescription of exercise training.
Methods
Protocol and registration
This review was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [16] and was prospectively registered on the International Prospective Register of Systematic Reviews (PROSPERO) as CRD42015019577.
Search strategy
Electronic searching of five databases (PubMed, MEDLINE, Scopus, BIOSIS, and Web of Science) was performed from the earliest date available up to July 2018. Independent variable search terms were ‘multicomponent training’, ‘multicomponent exercise’, ‘circuit training’, ‘circuit resistance training’, ‘combined exercise training’, ‘combined training’, ‘multi-modal exercise training’, ‘same-session exercise’, ‘concurrent training’, and ‘concurrent exercise’. Dependent variable search terms were ‘functional fitness’, ‘functional performance’, ‘physical performance’, ‘quality of life’, ‘functional decline’, ‘aerobic fitness’, ‘strength’, and ‘power’. Independent variable search terms were combined with dependent variable search terms using the ‘AND’ operator, giving a total of 80 search combinations. Reference lists from retrieved studies were also examined for potentially eligible papers.
Inclusion criteria
Study design
This review considered only original research articles, published in English. Randomised and non-randomised controlled trials were included, while uncontrolled, cross-sectional, and single-group, pre–post studies were excluded.
Participants
Only studies involving healthy, community-dwelling participants aged > 50 years were included. We defined an age of 50 years as our cut-off point for inclusion as there are clear physical and physiological declines beyond this age in adults [17, 18]. In addition, evidence suggests that physical capability in midlife is predictive of physical performance in later life [19], implying that earlier intervention at ~ 50 years of age can have positive long-term implications. It is acknowledged, however, that the use of an arbitrary threshold implies synonymy between chronological and biological age; yet, there remains no consensus on when old age begins, suggesting that any arbitrary definition is likely to be imperfect [20].
Studies involving participants with non-communicable disease (e.g., cardiovascular disease, type 2 diabetes mellitus, cancers, and chronic obstructive pulmonary disease) or who were being prescribed a specific pharmacological treatment were excluded. Studies were not automatically excluded if participants were labelled as an alternative population group (e.g. obese), as these were considered an extension of a healthy population rather than an alternative clinical group. For example, the study of Stewart et al. [21] categorised their participants as having ‘untreated milder forms of hypertension’, so was suitable for inclusion.
Training interventions
To be considered for inclusion in this systematic review, studies were required to include at least one combined exercise training group and a comparator group of either (1) no-exercise control; (2) endurance training only; or (3) strength training only. To be considered ‘combined training’ each training session within the intervention had to contain discrete, standalone activities of (1) endurance training and (2) strength training. Endurance training was defined as exercise involving large muscle groups in dynamic activities that result in substantial increases in heart rate and energy expenditure and was not limited to any specific modes of exercise [22]. Strength training was defined as any muscle-strengthening activities including where participants worked against or moved an external resistance (e.g., free weights, weight machines, elastic resistance bands, body weight exercises) [22, 23]. Interventions where training sessions contained additional training elements targeting improvement in other fitness components (e.g., balance, flexibility, coordination) were not excluded if endurance and strength training activities were present in each session. Activities prescribed as ‘warm up’ or ‘recovery’ were not considered. Training interventions were required to be a minimum of 2 weeks in duration with all training sessions supervised to ensure the fidelity of the intervention as previous work has suggested that exercise adherence is generally higher in supervised programmes [24] and observed effects may be greater when training is supervised [25]. Studies involving nutritional interventions were only included if there was a combined training and a comparator group (described previously) which were not exposed to these interventions. Interventions labelled as ‘circuit training’ typically involving subjects performing resistance exercises interspersed with aerobic exercises were not included as circuit training and combined training are two discrete training modes.
Outcome measures
As an important aim of the present work was to generate practically relevant information for clinicians and practitioners in the applied environment, we sought to provide meaningful context to our results by reporting raw mean differences rather than standardised mean differences (SMD). The SMD can be difficult to interpret on a practical level [26] and the use of the standard deviation (SD) to standardise each effect can introduce heterogeneity that is unrelated to any real differences in the effect between studies [27]. The SMD is often used when studies assess the same outcome but measure it in a variety of ways; however, it may be inappropriate to use different, albeit similar tests of physical fitness interchangeably unless equivalence has been demonstrated. This is because successful performance may be determined by different physiological parameters and issues such as reliability and test sensitivity may vary [28].
Accordingly, we selected a range of specific outcome measures to assess the effectiveness of same-session combined exercise training with our decision influenced by the personal experience of the authors as well as the desire to include functionally relevant measures of fitness with limited floor and ceiling effects. Studies required to contain at least one of the following outcome measures to be included in this meta-analysis: (1) peak oxygen uptake (VO2peak) or maximal oxygen uptake (VO2max), assessed via maximal incremental test—associated with the ability to maintain independent function and prevent disability [4, 5]; (2) six-minute walk test (6MWT)—a valid and reliable measure of physical endurance associated with self-reported functional ability with performance determined by leg strength and power [29, 30]; (3) 8-ft timed up-and-go (TUG)—a composite measure of performance related to dynamic balance and mobility measured over a distance of 8 ft [31]. We selected the 8-ft distance as previous authors [31] have suggested that this version of the test can be more feasibility administered in a home setting, is simpler for participants to perform, and has better sensitivity than alternative versions [28]; (4) 30-s chair stand—a valid measure of lower body muscle functioning [32] capable of detecting change in functional capacity in older adults [33]. Assessment of functional fitness (e.g., 30-s chair stand, TUG) provides a composite measure of physical capability as successful performance on these tests is determined by several factors [34], providing a functionally relevant and ecologically valid assessment of fitness.
Study selection
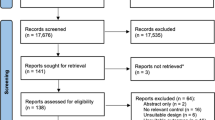
To identify relevant studies, all records were screened independently for eligibility by two authors (CH and KLW) with any disagreements resolved by a third reviewer (MW). Papers that were clearly not relevant were removed from the database list before assessing all other titles and abstracts using our pre-determined inclusion and exclusion criteria. Following this, full-text papers, including reviews, were then collected for evaluation. When full texts were not available, the corresponding author was contacted. After removal of duplicates and elimination of papers based on title and abstract screening, there were 413 studies remaining (Fig. 1). After evaluation of full texts, there were 27 papers that met our inclusion criteria and were, therefore, included in the meta-analysis.
Data extraction
Data were extracted from eligible studies into a custom-made spreadsheet by the lead author with a second investigator checking the extracted data for accuracy (KW/SM). Mean and standard deviation of pre-training and post-training values along with sample size from the combined and comparator groups were extracted for each outcome measure. In studies where standard deviations were not reported, they were calculated using the standard errors or confidence intervals provided [35]. Data for potential moderator variables that could reasonably influence the overall effect of training on changes in fitness were also extracted. This included both participant (mean age, proportion of males, and baseline fitness) and intervention characteristics [intervention duration (weeks) and training frequency (sessions per week)]. Where intervention duration was expressed as months, this value was converted to weeks based on 1 month being equal to 4.3 weeks. Attempts were made to contact corresponding authors via e-mail to obtain further information or clarity when needed.
In studies with multiple data sets [36,37,38,39,40,41] where more than one comparison was possible (e.g., combined versus no-exercise control and combined versus endurance only) sample size was halved where necessary to avoid double counting in analysis [42]. Several studies [36, 43,44,45] reported data at several time points or after a period of detraining; in all instances, pre- and post-intervention data only were analysed. In studies where there were more than one combined training group performing the same exercise training, but, in a different order (i.e., endurance training first or strength training first within a session) [37, 46, 47], data were combined using procedures as described in the Cochrane handbook [35].
Assessment of study quality
Methodological risk of bias was assessed by two investigators (CH and KW) according to the Cochrane Collaboration’s tool for assessing risk of bias [35]. Any disagreements were resolved by discussion to reach consensus.
Data analysis
Meta-analysis
All data analyses were performed using Comprehensive Meta-Analysis software, version 3 (Biostat Inc., Englewood, NJ, USA). Separate random-effects meta-analyses were performed to determine the pooled effect of change in each outcome measure for (1) combined training compared with no-exercise control, (2) combined training compared with endurance training only, and (3) combined training compared to strength training only. The precision of the pooled effect was expressed as 95% confidence limits (CL), calculated using the Knapp and Hartung approach [48]. To provide a real-world, measure for practical/clinical interpretation of our results, we evaluated the effects for each outcome measure against pre-specified thresholds for small, moderate, and large effects [49]. As robust clinical anchors for our outcome measures remain to be determined in this population, magnitude of effects were defined as standardised mean differences of 0.2, 0.6, and 1.2 between-subject standard deviations (SD) for small, moderate, and large effects, respectively [50]. The SD of the pooled baseline values was used for this purpose, as the post-intervention SD can be inflated by individual differences in response to an intervention [51]. Magnitude thresholds for small, moderate, and large effects, respectively, were: VO2peak, 0.6, 1.8, and 3.6 mL kg−1 min−1; 6MWT, 12.5, 37.6, and 75.1 m; TUG, 0.2, 0.5, and 1.1 s; 30 s-sit-to-stand, 1, 3, and 6 repetitions (expressed as an integer as partial repetitions are not possible).
Using the pooled effect for each outcome measure, together with its uncertainty (i.e. the confidence interval), the probability of the true effect being trivial, beneficial, or harmful was calculated, and then interpreted using the following scale: < 0.5%, most unlikely or almost certainly not; 0.5–5%, very unlikely; 5–25%, unlikely or probably not; 25–75%, possibly; 75–95%, likely; 95–99.5%, very likely; > 99.5%, most likely [50]. Effects were evaluated clinically, given that exercise interventions can be potentially harmful (i.e., reduce physical performance and functional capacity) as well as beneficial to individuals, and were considered unclear if the chance of benefit (improved physical performance) was high enough to warrant use of the intervention but with an unacceptable risk of harm (reduced physical performance). An odds ratio of benefit to harm of < 66 was used to identify such unclear effects. Between-study heterogeneity [Tau (τ)] was expressed as an SD [52], calculated using DerSimonian and Laird’s generalised method of moments [53], and doubled to interpret its magnitude against the above scale of effect sizes [54].
Meta-regression
Meta-regression was performed to explore the effect of five putative moderator variables which could reasonably influence the effect of training on fitness. These continuous variables were baseline fitness, intervention duration, weekly training frequency, age, and maleness (i.e., the proportion of males in the study sample). The modifying effects of these variables were calculated as the effect of two SDs (i.e., the difference between a typically low and a typically high value) and were evaluated non-clinically [50]. Meta-regression was performed only when there were > 10 data sets [35].
Publication bias
Publication bias was assessed using Egger’s test to evaluate asymmetry of funnel plots [55]. However, caution in interpreting these results is warranted when there are less than ten studies in the meta-analysis as the power of the test is too low to distinguish chance from real asymmetry [35].
Results
Study characteristics
Final analysis included 27 studies involving 1346 subjects with a mean age of 68.8 ± 5.9 years. There were 15 studies included in the meta-analysis of VO2peak [21, 38,39,40, 46, 47, 56,57,58,59,60,61,62,63,64], nine studies for 6MWT [37, 41, 43,44,45, 65,66,67,68], four for TUG [41, 45, 65, 69], and seven for 30-s chair stand [36, 41, 45, 46, 65, 68, 70]. Overall, there were 24 studies which included a no-exercise control group, six studies including a strength training only group, and seven studies including an endurance training only group. Individual study characteristics and intervention characteristics can be found in Table 1 and the electronic supplementary material (ESM) available with this article (ESM 1).
Risk of bias assessment
A summary of risk of bias assessment is presented in Fig. 2. Individual study-level data can be found in the electronic supplementary material available with this article (ESM 2). Risk of bias was predominantly low or unclear across all domains.
Effect of combined exercise training on VO2peak
The meta-analysed effect of combined training, when compared to no-exercise controls (Fig. 3), was a most likely moderate (possibly large) beneficial effect on VO2peak (3.6 mL kg−1 min−1; ± 95% confidence limits 0.8 mL kg−1 min−1). Between-study heterogeneity (τ) was ± 0.9 mL kg−1 min−1 (small magnitude). Egger’s coefficient was − 1.89 (95% CI − 3.27 to − 0.51; p = 0.01). Of the five moderator variables selected, meta-regression analysis revealed a greater additional beneficial effect (possibly moderate) for studies with a higher proportion of female participants (2.1 mL kg−1 min−1; ± 1.8 mL kg−1 min−1) and a likely small additional benefit for typically shorter training programmes (1.6 mL kg−1 min−1; ± 1.5 mL kg−1 min−1). The effect of all other putative modifiers was unclear. When compared against endurance training only (Fig. 4), the meta-analysed effect of combined training was a possibly small beneficial effect on VO2peak (0.8 mL kg−1 min−1; ± 1.0 mL kg−1 min−1). Between-study heterogeneity was trivial (± 0 mL kg−1 min−1) and Egger’s coefficient was − 0.41 (− 1.62 to 0.80; p = 0.36).
Effect of combined training on 6MWT performance
When compared to no-exercise controls (Fig. 5), there was a very likely small beneficial effect for combined training on 6MWT performance (29.6 m; ± 20.5 m). Between-study heterogeneity (τ) was small (± 18.7 m), while Egger’s coefficient was 1.26 (− 2.10 to 4.63; p = 0.39). The meta-analysed effect of combined training versus strength training only (Fig. 6) was unclear (5.8 m; ± 20.2 m) with between-study heterogeneity trivial (± 0 m) and Egger’s coefficient 0.65 (− 1.82 to 3.12; p = 0.18).
Effect of combined training on TUG performance
The meta-analysed effect of combined training compared with no-exercise controls (Fig. 7) was a likely moderate beneficial effect on timed up-and-go performance (0.8 s; ±0.4 s). Between-study heterogeneity (τ) was small (± 0.2 s), while Egger’s coefficient was − 1.63 (− 7.73 to 4.47); p = 0.37. Compared with strength training only (Fig. 8), the effect for combined training was unclear (0.3 s; ± 0.6 s). Assessment of publication bias was not possible for combined training versus strength training only as there were only two data sets. Between-study heterogeneity (τ) was trivial (± 0 s).
Effect of combined training on 30-s chair stand test performance
Compared to no-exercise controls, the meta-analysed effect of combined training (Fig. 9) was a most likely small (possibly moderate) beneficial effect on 30-s chair stand performance (3.1 repetitions; ± 1.3 repetitions). Between-study heterogeneity (τ) was moderate (± 1.5 repetitions) and Egger’s coefficient was − 0.17 (− 4.37 to 4.04; p = 0.92). Compared with strength training only (Fig. 10), there was a possibly small beneficial effect for combined training (1.1; ± 0.5 repetitions). For combined training versus strength training only, there were only two data sets, so assessment of publication bias was not possible. Between-study heterogeneity (τ) was trivial (± 0 repetitions).
Discussion
Given the proposed benefit of training programmes involving a combination of endurance and strength training on physical performance in older adults, we sought to systematically review and quantify the effects of same-session combined training on measures of fitness in adults aged over 50 years. Our results demonstrate clear and functionally relevant beneficial effects for combined training on VO2peak, 6MWT, TUG, and 30-s chair stand when compared with no-exercise controls. In addition, there was a small beneficial effect for combined training when compared to endurance training only for VO2peak.
The data reported in this investigation confirm the findings of previous experimental studies [21, 38, 39], demonstrating that combined training—advocated as a method for simultaneously improving cardiorespiratory and muscular fitness [12]—is an effective training strategy for improving VO2peak. The meta-analysed effect reported here is comparable to previous meta-analyses reporting mean improvements of 3.78 mL kg−1 min−1 [71] and 3.5 mL kg−1 min−1 [72] following endurance training in older adults, suggesting that combined training may be as effective for cardiorespiratory fitness improvement as traditional endurance training alone. Moreover, when compared with endurance training alone, combined training had a possibly small beneficial effect on VO2peak, thereby suggesting that combined training may be a more efficacious training strategy for improving VO2peak in this population. Although the present investigation has not sought to understand the mechanisms of adaptation underlying training-induced changes, central and peripheral adaptations to endurance training—such as improved delivery, utilisation and extraction of oxygen—may explain increased VO2peak [73]. In the context of combined training, it may be that the inclusion of strength training provides an additive benefit as previous investigations have reported improvements in cardiorespiratory fitness following strength training [74]—potentially mediated by increases in capillary density and mitochondrial enzyme activity [75, 76]. Furthermore, improvements in lower body strength may lead to increased time to exhaustion on an incremental exercise test, thereby increasing observed VO2peak [77].
The effect of combined training on VO2peak was greater for female participants, a finding in contrast to previous work, indicating that the observed response following endurance training in older adults is not moderated by sex [78]. Our findings may be related to lower cardiorespiratory fitness typically observed in females compared to males [18] as fitness improvements are typically greater for those with lower baseline fitness. For example, previous work has reported possibly small and likely moderate greater beneficial effects for participants with lower baseline fitness following endurance training and high-intensity interval training (HIT), respectively [79]. However, we observed no clear modifying effect for baseline fitness, a finding which may have been influenced by the small number and heterogeneity of studies included in our meta-regression. The complex interplay of participant (e.g., age, sex, and baseline fitness) and intervention characteristics (e.g., exercise prescription and adherence), which may influence training response, as well as between-study differences in these characteristics, also likely contributes to the observed findings. The small number of included studies means that considerable uncertainty remains, with more work needed to understand sex-specific responses to combined exercise training.
Meta-regression also demonstrated a likely small influence for shorter training programmes; a potentially meaningful finding with important practical implications as training programmes requiring a reduced time commitment may be more appealing to potential exercisers [80]. Previous work has shown that shorter duration training programmes can induce improvements in cardiorespiratory fitness that are comparable, or even greater than those following longer duration programmes [81] with gains in VO2max similar following 8–12 or 50 weeks of training [71]. Exercise programming variables (e.g., exercise intensity, volume, and progression) represent key mediators of training response and likely play a more meaningful role than exercise programme duration in determining training adaptation [82, 83]. Between-study differences in these variables contribute to the findings reported in this meta-analysis and make it difficult to draw conclusive inference. There remains a need for future work to explore the modifying effects of programming variables on training response following combined training in older adults.
As well as cardiorespiratory fitness, maintaining muscular fitness is a primary aim of exercise training interventions in older adults because of the role which it plays in determining functional capacity with ageing [7]. Multiple methods of assessment should be used to evaluate training-induced changes in muscular fitness [84] with functional fitness tests recommended to evaluate performance in the context of the activities of daily living [85]. Typically, these measures provide a composite measure of physical capability as successful performance on these tests is determined by several components of fitness [29, 34], thereby providing a more functionally relevant and ecologically valid assessment of physical performance. The present investigation has reported small-to-moderate beneficial effects for combined training compared with no-exercise controls for 6MWT, TUG, and 30-s chair stand. These are important and clinically relevant findings as low levels of physical capability can limit older adults ability to perform the basic activities of daily living [86]. Interestingly, data also indicated a possibly small beneficial effect for combined training compared with strength training only for 30-s chair stand. However, caution is warranted in interpreting this finding as only two studies were included in the analysis.
The observed effects in this meta-analysis are greater than those reported in a previous systematic review by Baker and colleagues [87] who found that multi-modal exercise (i.e., training programmes consisting of aerobic training, strength training, and balance training) induces small and inconsistent effects on measures of functional fitness. Previous work has also demonstrated that both endurance training [88] and resistance training [9] performed in isolation are effective at eliciting positive adaptations in these measures of functional fitness. For example, Kalapotharakos and colleagues [88] evaluated the effect of a 12-week progressive high-intensity endurance training programme, performed three times per week and reported a 17% increase in 6WMT distance. This improvement was greater than the effect reported in this meta-analysis; however, the baseline fitness of the participants in this study was lower than in the present investigation. It is important to note that comparisons with previous studies are confounded by differences in participant characteristics (e.g., baseline fitness) and exercise prescription, which likely contribute to discordance between findings. More experimental work is needed to evaluate the optimal training strategy for inducing improvements in measures of functional fitness.
A range of intervention studies have documented that strength training is an effective approach for improving muscular fitness and functional performance in older adults [9, 89]. For example, lower extremity strength gain is associated with chair rise performance, gait speed, and mobility tasks [90], while strength training can improve muscle power of the lower body muscle groups relevant for carrying out daily functional tasks [91]. Several meta-analyses have extended these findings, thereby reinforcing the effectiveness of strength training for improving muscular fitness in older adults [92,93,94]. It, therefore, seems likely that performing strength training within a combined training programme contributes to the observed improvements reported in this investigation with these changes mediated by a range of morphological and neurological adaptations [95]. These findings have important practical implications in older adults as functional fitness is associated with reduced risk of disability and enhanced functional independence in older adults [96, 97]. In the wider context of exercise training in older adults, the observed findings are largely unsurprising based on the principle of training specificity [98, 99]. Both endurance and strength training are effective approaches for improving cardiorespiratory and muscular fitness, respectively, in older adults. Theoretically, therefore, it makes sense that the combination of these training modes would elicit improvements in both cardiorespiratory and muscular fitness.
The pooled effects presented in this meta-analysis provide an overall quantification of the effects of same-session combined training which can be used for the comparison and assessment of superiority with alternative modes of exercise training in subsequent investigations. As older adults have a clear need to maintain muscular and cardiorespiratory fitness, exercise strategies which are able to induce improvements in both of these physiological systems within the same training session may be a more efficient, and thereby, attractive proposition for potential exercisers. One potential comparator exercise mode is high-intensity interval training (HIT). Conceptually, this comparison makes sense as HIT is also capable of inducing improvements in both cardiorespiratory and muscular fitness with an exercise stimulus delivered within a single exercise session [8]. While long-term studies evaluating HIT in older adults are limited, current findings are encouraging with previous investigation demonstrating that HIT favours older and less fit individuals [79] with significant improvements in fitness possible after short-duration training programmes [8]. Future experimental work should continue to evaluate and compare the short- and long-term effects of alternate training strategies in this population to allow comprehensive evidence-based exercise recommendations to evolve.
The findings presented in the current investigation should be interpreted with caution for several reasons. First, the inclusion of only healthy community-dwelling adults aged over 50 years limits the generalisability of these results as they should not be extrapolated more widely to include frail elderly or adults with chronic long-term conditions. As considerable interindividual variation exists throughout the healthy ageing process [100], including participants characterised as non-healthy would have added further heterogeneity to this work, further limiting the impact of potential findings. However, those individuals characterised as ‘at risk’ or who are ‘non-healthy’ have potential to benefit most from therapeutic interventions such as exercise training and there remains a need for further work in these population groups. Although we defined a similar age cut-off to previous investigations [91], included studies represented a broad age range of participants. A lack of available data meant that it was not possible to perform specific analyses of typically defined discrete age categories (e.g., 50–64 years or 65–80 years), though it should be noted that there was no clear effect for age when included within our meta-regression for VO2peak. In addition, only one study included participants with a mean age over 80 years, an important consideration because of the projected future growth of this population group [101].
Second, several analyses presented are limited by the small numbers of eligible studies, which, combined with the between-study heterogeneity, may have affected the magnitude of observed effects and the uncertainty of these effects (e.g., the width of the reported CLs). One of the primary factors explaining the small number of included studies is the large variation in outcomes assessed and measurement tools used by different authors and research groups to evaluate training interventions. As one of the primary aims of this work was to provide practically relevant estimates of mean effects, we included only studies which contained our pre-determined specific outcome measures. In doing so, we acknowledge the exclusion of a considerable body of research. Future work is needed to fully appraise the equivalence of different measurement tools which aim to evaluate the same physical component by evaluating and comparing physical and physiological determinants of performance as well as measures of reliability, validity, and sensitivity [102]. Although challenging to implement, standardised recommendations for a battery of physical capacity tests to evaluate training interventions in older adults would also aid future attempts at synthesising research findings.
Finally, the practical implications of this work are limited by the wide variability of training programmes and incomplete reporting within the included studies. Exercise volume and intensity are both important mediators of training adaptation for both endurance and strength training [103, 104], yet the reporting of these data was inconsistent or incomplete across a number of included studies. As such, it was not possible to extract and fully evaluate the effects of exercise intensity on outcomes following combined training. While the authors of systematic reviews and meta-analyses can attempt to find further information about the study characteristics, this is a time-consuming and often ineffective process [105]. As such, the present meta-analysis is in agreement with Straight et al. [91] in calling for standardised reporting of exercise training protocols to enable researchers to fully quantify the effects of training in future meta-analyses. This should include the presentation of training programming variables (e.g., training intensity, volume, frequency, and duration) as well as information about the fidelity of the intervention [106]. While the present findings provide support for the effectiveness of same-session combined exercise training as a strategy to induce functionally relevant fitness improvements, a lack of studies including a comparator exercise mode limits the potential value of the present work and makes it difficult to draw inference about the effectiveness of this exercise approach compared with the other exercise modes typically utilised in this population (e.g., endurance or strength training). The finding that combined training improves fitness compared with no exercise is largely unsurprising and further experimental work is needed to establish superiority between exercise training modes in this population.
Conclusions
The findings of the present meta-analysis provide further evidence supporting the application of combined exercise training as a strategy for fitness improvement in adults aged over 50 years. The quantitative mean effects presented in this investigation may help practitioners and clinicians to make informed decisions relating to future training prescription in this population. However, despite some encouraging experimental findings and the data presented in this systematic review, considerable uncertainty remains regarding the effectiveness of same-session combined exercise training compared with endurance or strength training performed alone. Further experimental work is needed to address this deficiency of knowledge and to establish superiority between these training approaches in this population. Future investigations should also seek to understand the modifying effects of exercise programming variables (e.g., volume and intensity) on training outcomes to optimise the prescription of combined training interventions.
Data Availability
The data sets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Kodama S, Saito K, Tanaka S et al (2009) Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA 301:2024–2035. https://doi.org/10.1001/jama.2009.681
Ruiz JR, Sui X, Lobelo F et al (2008) Association between muscular strength and mortality in men: prospective cohort study. BMJ 337:a439. https://doi.org/10.1136/bmj.a439
Kim Y, White T, Wijndaele K et al (2018) The combination of cardiorespiratory fitness and muscle strength, and mortality risk. Eur J Epidemiol 301:1–12. https://doi.org/10.1007/s10654-018-0384-x
Morey MC, Pieper CF, Cornoni-Huntely J (1998) Physical fitness and functional limitations in community-dwelling older adults. MSSE 30:715–723. https://doi.org/10.1097/00005768-199805000-00012
Paterson DH, Govindasamy D, Vidmar M et al (2004) Longitudinal study of determinants of dependence in an elderly population. J Am Geriatr Soc 52:1632–1638. https://doi.org/10.1111/j.1532-5415.2004.52454.x
Hairi NN, Cumming RG, Naganathan V et al (2010) Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: the concord health and ageing in men project. J Am Geriatr Soc 58:2055–2062. https://doi.org/10.1111/j.1532-5415.2010.03145.x
Manini TM, Clark BC (2012) Dynapenia and aging: an update. J Gerontol Ser A Biol Sci Med Sci 67:28–40. https://doi.org/10.1093/gerona/glr010
Hurst C, Weston KL, Weston M (2018) The effect of 12 weeks of combined upper- and lower-body high-intensity interval training on muscular and cardiorespiratory fitness in older adults. Aging Clin Exp Res. https://doi.org/10.1007/s40520-018-1015-9
Stec MJ, Thalacker-Mercer A, Mayhew DL et al (2017) Randomized, four-arm, dose-response clinical trial to optimize resistance exercise training for older adults with age-related muscle atrophy. Exp Gerontol 99:98–109. https://doi.org/10.1016/j.exger.2017.09.018
Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA et al (2009) Exercise and physical activity for older adults. Med Sci Sports Exerc 41:1510–1530. https://doi.org/10.1249/MSS.0b013e3181a0c95c
Nelson ME, Rejeski WJ, Blair SN et al (2007) Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation 116:1094–1105
Cadore EL, Izquierdo M (2013) How to simultaneously optimize muscle strength, power, functional capacity, and cardiovascular gains in the elderly: an update. Age 35:2329–2344. https://doi.org/10.1007/s11357-012-9503-x
Trudelle-Jackson E, Jackson AW (2018) Do older adults who meet 2008 physical activity guidelines have better physical performance than those who do not meet? J Geriatr Phys Ther 41:180–185. https://doi.org/10.1519/JPT.0000000000000118
Jefferis BJ, Sartini C, Lee I-M et al (2014) Adherence to physical activity guidelines in older adults, using objectively measured physical activity in a population-based study. BMC Public Health 14:382. https://doi.org/10.1186/1471-2458-14-382
Trost SG, Owen N, Bauman AE et al (2002) Correlates of adults’ participation in physical activity: review and update. Med Sci Sports Exerc 34:1996–2001. https://doi.org/10.1249/01.MSS.0000038974.76900.92
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097. https://doi.org/10.1371/journal.pmed.1000097
Deschenes DMR (2004) Effects of aging on muscle fibre type and size. Sports Med 34:809–824. https://doi.org/10.2165/00007256-200434120-00002
Fleg JL, Morrell CH, Bos AG et al (2005) Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation 112:674–682. https://doi.org/10.1161/CIRCULATIONAHA.105.545459
Cooper R, Strand BH, Hardy R et al (2014) Physical capability in mid-life and survival over 13 years of follow-up: British birth cohort study. BMJ 348:g2219–g2219. https://doi.org/10.1136/bmj.g2219
Tinkler A (1993) When does old age start? Int J Geriatr Psychiatry 8:711–716
Stewart KJ, Bacher AC, Turner KL et al (2005) Effect of exercise on blood pressure in older persons: a randomized controlled trial. Arch Intern Med 165:756–762. https://doi.org/10.1001/archinte.165.7.756
Howley ET (2001) Type of activity: resistance, aerobic and leisure versus occupational physical activity. Med Sci Sports Exerc 6:S364–S369
Garber CE, Blissmer B, Deschenes MR et al (2011) Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults. Med Sci Sports Exerc 43:1334–1359. https://doi.org/10.1249/MSS.0b013e318213fefb
Picorelli AMA, Pereira LSM, Pereira DS et al (2014) Adherence to exercise programs for older people is influenced by program characteristics and personal factors: a systematic review. J Physiother 60:151–156. https://doi.org/10.1016/j.jphys.2014.06.012
Lacroix A, Hortobágyi T, Beurskens R, Granacher U (2017) Effects of supervised vs. unsupervised training programs on balance and muscle strength in older adults: a systematic review and meta-analysis. Sports Med 47:2341–2361. https://doi.org/10.1007/s40279-017-0747-6
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR (2009) Introduction to meta-analysis. Wiley, Chichester
Hopkins WG Improving meta-analyses in sport and exercise science. Sportscience 22:11–17
Rolenz E, Reneker JC (2016) Validity of the 8-foot up and go, timed up and go, and activities-specific balance confidence scale in older adults with and without cognitive impairment. J Rehabil Res Dev 53:511–518. https://doi.org/10.1682/JRRD.2015.03.0042
Bean JF, Kiely DK, Leveille SG et al (2002) The 6-minute walk test in mobility-limited elders: what is being measured? J Gerontol Ser A Biol Sci Med Sci 57:M751–M756. https://doi.org/10.1093/gerona/57.11.m751
Rikli RE, Jones CJ (1998) The reliability and validity of a 6-minute walk test as a measure of physical endurance in older adults. J Aging Phys Act 6:363–375. https://doi.org/10.1123/japa.6.4.363
Rikli RE, Jones CJ (1999) Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act 7:129–161
Jones CJ, Rikli RE, Beam WC (1999) A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc. https://doi.org/10.1080/02701367.1999.10608028
Adamo DE, Talley SA, Goldberg A (2015) Age and task differences in functional fitness in older women: comparisons with senior fitness test normative and criterion-referenced data. J Aging Phys Act 23:47–54. https://doi.org/10.1123/JAPA.2012-0317
Lord SR, Murray SM, Chapman K et al (2002) Sit-to-stand performance depends on sensation, speed, balance, and psychological status in addition to strength in older people. J Gerontol Ser A Biol Sci Med Sci 57:M539–M543
Higgins JPT, Green S (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration
Douda HT, Kosmidou KV, Smilios I et al (2015) Community-based training–detraining intervention in older women: a five-year follow-up study. J Aging Phys Act 23:496–512. https://doi.org/10.1123/japa.2013-0241
Campos ALP, Del Ponte LS, Cavalli AS et al (2013) Effects of concurrent training on health aspects of elderly women. Braz J Kinanthropometry Hum Perform 15:437–447. https://doi.org/10.5007/1980-0037.2013v15n4p437
Cadore EL, Pinto RS, Lhullier FLR et al (2010) Physiological effects of concurrent training in elderly men. Int J Sports Med 31:689–697. https://doi.org/10.1055/s-0030-1261895
Ferketich AK, Kirby TE, Alway SE (1998) Cardiovascular and muscular adaptations to combined endurance and strength training in elderly women. Acta Physiol Scand 164:259–267. https://doi.org/10.1046/j.1365-201X.1998.00428.x
Delecluse C, Colman V, Roelants M et al (2004) Exercise programs for older men: mode and intensity to induce the highest possible health-related benefits. Preventive. https://doi.org/10.1016/j.ypmed.2004.03.023
Kim D-I, Lee DH, Hong S et al (2018) Six weeks of combined aerobic and resistance exercise using outdoor exercise machines improves fitness, insulin resistance, and chemerin in the Korean elderly: a pilot randomized controlled trial. Arch Gerontol Geriatr 75:59–64. https://doi.org/10.1016/j.archger.2017.11.006
Senn SJ (2009) Overstating the evidence—double counting in meta-analysis and related problems. BMC Med Res Methodol 9:697–697. https://doi.org/10.1186/1471-2288-9-10
King MB, Whipple RH, Gruman CA et al (2002) The performance enhancement project: improving physical performance in older persons. Arch Phys Med Rehabil 83:1060–1069. https://doi.org/10.1053/apmr.2002.33653
Wang R-Y, Wang Y-L, Cheng F-Y et al (2015) Effects of combined exercise on gait variability in community-dwelling older adults. Age 37:434–439. https://doi.org/10.1007/s11357-015-9780-2
Carvalho MJ, Marques E, Mota J (2009) Training and detraining effects on functional fitness after a multicomponent training in older women. Gerontology 55:41–48. https://doi.org/10.1159/000140681
Wilhelm EN, Rech A, Minozzo F et al (2014) Concurrent strength and endurance training exercise sequence does not affect neuromuscular adaptations in older men. Exp Gerontol 60:207–214. https://doi.org/10.1016/j.exger.2014.11.007
Engels HJ, Drouin J, Zhu W, Kazmierski JF (1998) Effects of low-impact, moderate-intensity exercise training with and without wrist weights on functional capacities and mood states in older adults. Gerontology 44:239–244. https://doi.org/10.1159/000022018
Knapp G, Hartung J (2003) Improved tests for a random effects meta-regression with a single covariate. Stat Med 22:2693–2710. https://doi.org/10.1002/sim.1482
Batterham AM, Hopkins WG (2006) Making meaningful inferences about magnitudes. Int J Sports Physiol Perform 1:50–57
Hopkins WG, Marshall SW, Batterham AM, Hanin J (2009) Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc 41:3–13. https://doi.org/10.1249/MSS.0b013e31818cb278
Atkinson G, Batterham AM (2015) True and false interindividual differences in the physiological response to an intervention. Exp Physiol 100:577–588. https://doi.org/10.1113/EP085070
Higgins JPT (2008) Commentary: Heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol 37:1158–1160. https://doi.org/10.1093/ije/dyn204
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188. https://doi.org/10.1016/0197-2456(86)90046-2
Smith TB, Hopkins WG (2011) Variability and predictability of finals times of elite rowers. Med Sci Sports Exerc 43:2155–2160. https://doi.org/10.1249/MSS.0b013e31821d3f8e
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634. https://doi.org/10.1136/bmj.315.7109.629
Cress ME, Thomas DP, Johnson J et al (1991) Effect of training on VO2max, thigh strength, and muscle morphology in septuagenarian women. Med Sci Sports Exerc 23:752–758
Puggaard L (2003) Effects of training on functional performance in 65, 75 and 85 year-old women: experiences deriving from community based studies in Odense, Denmark. Scand J Med Sci Sports 13:70–76
Villareal DT, Chode S, Parimi N et al (2011) Weight loss, exercise, or both and physical function in obese older adults. NEJM 364:1218–1229. https://doi.org/10.1056/NEJMoa1008234
Cress ME, Buchner DM, Questad KA et al (1999) Exercise: effects on physical functional performance in independent older adults. J Gerontol Ser A Biol Sci Med Sci 54:M242–M248. https://doi.org/10.1093/gerona/54.5.M242
Park H, Kim KJ, Komatsu T et al (2008) Effect of combined exercise training on bone, body balance, and gait ability: a randomized controlled study in community-dwelling elderly women. J Bone Miner Metab 26:254–259. https://doi.org/10.1007/s00774-007-0819-z
Park J, Nakamura Y, Kwon Y, Park H (2010) The effect of combined exercise training on carotid artery structure and function, and vascular endothelial growth factor (VEGF) in obese older women. Jpn J Phys Fit Sports Med 59:495–504. https://doi.org/10.7600/jspfsm.59.495
Kwon Y, Park S, Kim E, Park J (2008) The effects of multi-component exercise training on VO2max, muscle mass, whole bone mineral density and fall risk in community-dwelling elderly women. Jpn J Phys Fit Sports Med 57:339–348. https://doi.org/10.7600/jspfsm.57.339
Park S-M, Kwak Y-S, Ji J-G (2015) The effects of combined exercise on health-related fitness, endotoxin, and immune function of postmenopausal women with abdominal obesity. J Immunol Res 2015:1–8. https://doi.org/10.1155/2015/830567
Schaun MI, Dipp T, Silva Rossato J et al (2011) The effects of periodized concurrent and aerobic training on oxidative stress parameters, endothelial function and immune response in sedentary male individuals of middle age. Cell Biochem Funct 29:534–542. https://doi.org/10.1002/cbf.1781
Marques EA, Mota J, Machado L et al (2011) Multicomponent training program with weight-bearing exercises elicits favorable bone density, muscle strength, and balance adaptations in older women. Calcif Tissue Int 88:117–129. https://doi.org/10.1007/s00223-010-9437-1
Rubenstein LZ, Josephson KR, Trueblood PR et al (2000) Effects of a group exercise program on strength, mobility, and falls among fall-prone elderly men. J Gerontol Ser A Biol Sci Med Sci 55:M317–M321
Marques E, Carvalho J, Soares JMC et al (2009) Effects of resistance and multicomponent exercise on lipid profiles of older women. Maturitas 63:84–88. https://doi.org/10.1016/j.maturitas.2009.03.003
Desjardins-Crepeau L, Berryman N, Fraser S et al (2016) Effects of combined physical and cognitive training on fitness and neuropsychological outcomes in healthy older adults. CIA 11:1287–1299. https://doi.org/10.2147/CIA.S115711
Timmons JF, Minnock D, Hone M et al (2018) Comparison of time-matched aerobic, resistance, or concurrent exercise training in older adults. Scand J Med Sci Sports 28:2272–2283. https://doi.org/10.1111/sms.13254
García-Pinillos F, Laredo-Aguilera JA, Muñoz-Jiménez M, Latorre-Román PA (2017) Effects of 12-week concurrent high-intensity interval strength and endurance training programme on physical performance in healthy older people. J Strength Cond Res 1–25. https://doi.org/10.1519/JSC.0000000000001895
Huang G, Wang R, Chen P et al (2016) Dose–response relationship of cardiorespiratory fitness adaptation to controlled endurance training in sedentary older adults. Eur J Prev Cardiol 23:518–529. https://doi.org/10.1177/2047487315582322
Green JS, Crouse SF (1995) The effects of endurance training on functional capacity in the elderly: a meta-analysis. Med Sci Sports Exerc 27:920–926
Daussin FN, Zoll J, Dufour SP et al (2008) Effect of interval versus continuous training on cardiorespiratory and mitochondrial functions: relationship to aerobic performance improvements in sedentary subjects. AJP Regulat Integr Comp Physiol 295:R264–R272. https://doi.org/10.1152/ajpregu.00875.2007
Ozaki H, Loenneke JP, Thiebaud RS, Abe T (2013) Resistance training induced increase in VO2max in young and older subjects. Eur Rev Aging Phys Act 10:107–116. https://doi.org/10.1007/s11556-013-0120-1
Frank P, Andersson E, Pontén M et al (2015) Strength training improves muscle aerobic capacity and glucose tolerance in elderly. Scand J Med Sci Sports 26:764–773. https://doi.org/10.1111/sms.12537
Frontera WR, Meredith CN, O’Reilly KP, Evans WJ (1990) Strength training and determinants of VO2max in older men. J Appl Physiol 68:329–333
Karavirta L, Häkkinen K, Kauhanen A et al (2011) Individual responses to combined endurance and strength training in older adults. Med Sci Sports Exerc 43:484–490. https://doi.org/10.1249/MSS.0b013e3181f1bf0d
Kohrt WM, Malley MT, Coggan AR et al (1991) Effects of gender, age, and fitness level on response of VO2max to training in 60–71 year olds. J Appl Physiol 71:2004–2011
Milanovic Z, Sporiš G, Weston M (2015) Effectiveness of high-intensity interval training (HIT) and continuous endurance training for VO2max improvements: a systematic review and meta-analysis of controlled trials. Sports Med 45:1469–1481. https://doi.org/10.1007/s40279-015-0365-0
Vollaard NBJ, Metcalfe RS (2017) Research into the health benefits of sprint interval training should focus on protocols with fewer and shorter sprints. Sports Med 380:247–249. https://doi.org/10.1007/s40279-017-0727-x
Murias JM, Kowalchuk JM, Paterson DH (2010) Time course and mechanisms of adaptations in cardiorespiratory fitness with endurance training in older and young men. J Appl Physiol 108:621–627. https://doi.org/10.1152/japplphysiol.01152.2009
Shephard RJ (1968) Intensity, duration and frequency of exercise as determinants of the response to a training regime. Int Z Angew Physiol Einschl Arbeitsphysiol 26:272–278. https://doi.org/10.1007/BF00695115
Wenger HA, Bell GJ (1986) The interactions of intensity, frequency and duration of exercise training in altering cardiorespiratory fitness. Sports Med 3:346–356. https://doi.org/10.2165/00007256-198603050-00004
Buckner SL, Jessee MB, Mattocks KT et al (2016) Determining strength: a case for multiple methods of measurement. Sports Med 47:193–195. https://doi.org/10.1007/s40279-016-0580-3
Weston M, Weston KL, Prentis JM, Snowden CP (2016) High-intensity interval training (HIT) for effective and time-efficient pre-surgical exercise interventions. Perioper Med 5:1380–1389. https://doi.org/10.1186/s13741-015-0026-8
Wennie Huang W-N, Perera S, VanSwearingen J, Studenski S (2010) Performance measures predict onset of activity of daily living difficulty in community-dwelling older adults. J Am Geriatr Soc 58:844–852. https://doi.org/10.1111/j.1532-5415.2010.02820.x
Baker MK, Atlantis E, Fiatarone Singh MA (2007) Multi-modal exercise programs for older adults. Age Ageing 36:375–381. https://doi.org/10.1093/ageing/afm054
Kalapotharakos VI, Michalopoulos M, Strimpakos N et al (2006) Functional and neuromotor performance in older adults. Am J Phys Med Rehabil 85:61–67. https://doi.org/10.1097/01.phm.0000179479.30543.1c
Farinatti PTV, Geraldes AAR, Bottaro MF et al (2013) Effects of different resistance training frequencies on the muscle strength and functional performance of active women older than 60 years. J Strength Cond Res 27:2225–2234. https://doi.org/10.1519/jsc.0b013e318278f0db
Chandler JM, Duncan PW, Kochersberger G (1998) Is lower extremity strength gain associated with improvement in physical performance and disability in frail, community-dwelling elders? Arch Phys Med Rehabil 79:24–30. https://doi.org/10.1016/S0003-9993(98)90202-7
Straight CR, Lindheimer JB, Brady AO et al (2016) Effects of resistance training on lower-extremity muscle power in middle-aged and older adults: a systematic review and meta-analysis of randomized controlled trials. Sports Med 46:353–364. https://doi.org/10.1007/s40279-015-0418-4
Steib S, Schoene D, Pfeifer K (2010) Dose–response relationship of resistance training in older adults. Med Sci Sports Exerc 42:902–914. https://doi.org/10.1249/MSS.0b013e3181c34465
Borde R, Hortobágyi T, Granacher U (2015) Dose–response relationships of resistance training in healthy old adults: a systematic review and meta-analysis. Sports Med 45:1693–1720. https://doi.org/10.1007/s40279-015-0385-9
Peterson MD, Rhea MR, Sen A, Gordon PM (2010) Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res Rev 9:226–237. https://doi.org/10.1016/j.arr.2010.03.004
Folland JP, Williams AG (2007) The adaptations to strength training: morphological and neurological contributions to increased strength. Sports Med 37:145–168
Guralnik JM, Ferrucci L, Simonsick EM et al (1995) Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. NEJM 332:556–561. https://doi.org/10.1056/NEJM199503023320902
Misic MM, Rosengren KS, Woods JA, Evans EM (2007) Muscle quality, aerobic fitness and fat mass predict lower-extremity physical function in community-dwelling older adults. Gerontology 53:260–266. https://doi.org/10.1159/000101826
Holloszy JO, Coyle EF (1984) Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol Respir Environ Exerc Physiol 56:831–838
Ǻstrand PO, Rodahl K (1976) Textbook of work physiology: physiological bases of exercise, 3rd edn. McGraw-Hill, New York
Steves CJ, Spector TD, Jackson SHD (2012) Ageing, genes, environment and epigenetics: what twin studies tell us now, and in the future. Age Ageing 41:581–586. https://doi.org/10.1093/ageing/afs097
Office for National Statistics (2017) National population projections: 2016-based statistical bulletin. Available from https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationprojections/bulletins/nationalpopulationprojections/2016basedstatisticalbulletin
Currell K, Jeukendrup AE (2008) Validity, reliability and sensitivity of measures of sporting performance. Sports Med 38:297–316. https://doi.org/10.2165/00007256-200838040-00003
Gormley SE, Swain DP, High R et al (2008) Effect of intensity of aerobic training on VO2max. Med Sci Sports Exerc 40:1336–1343. https://doi.org/10.1249/MSS.0b013e31816c4839
Steib S, Schoene D, Pfeifer K (2010) Dose–response relationship of resistance training in older adults: a meta-analysis. Med Sci Sports Exerc 42:902–914. https://doi.org/10.1249/MSS.0b013e3181c34465
Tew GA, Brabyn S, Cook L, Peckham E (2016) The completeness of intervention descriptions in randomised trials of supervised exercise training in peripheral arterial disease. PLoS One 11:e0150869. https://doi.org/10.1371/journal
Taylor KL, Weston M, Batterham AM (2015) Evaluating intervention fidelity: an example from a high-intensity interval training study. PLoS One 10:e0125166–e0125168. https://doi.org/10.1371/journal.pone.0125166
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of human and animal rights
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hurst, C., Weston, K.L., McLaren, S.J. et al. The effects of same-session combined exercise training on cardiorespiratory and functional fitness in older adults: a systematic review and meta-analysis. Aging Clin Exp Res 31, 1701–1717 (2019). https://doi.org/10.1007/s40520-019-01124-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-019-01124-7