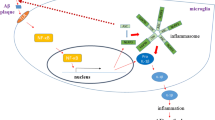
Abstract
Due to an increasingly aging population, Alzheimer disease (AD) represents a crucial issue for the healthcare system because of its widespread prevalence and the burden of its care needs. Several hypotheses on AD pathogenesis have been proposed and current therapeutical strategies have shown limited effectiveness. In the last decade, more evidence has supported a role for neuroinflammation and immune system dysregulation in AD. It remains unclear whether astrocytes, microglia and immune cells influence disease onset, progression or both. Amyloid-β peptides that aggregate extracellularly in the typical neuritic plaques generate a constant inflammatory environment. This causes a prolonged activation of microglial and astroglial cells that potentiate neuronal damage and provoke the alteration of the blood brain barrier (BBB), damaging the permeability of blood vessels. Recent data support the role of the BBB as a link between neuroinflammation, the immune system and AD. Hence, a thorough investigation of the neuroinflammatory and immune system pathways that impact neurodegeneration and novel exciting findings such as microglia-derived microvesicles, inflammasomes and signalosomes will ultimately enhance our understanding of the pathological process. Eventually, we should proceed with caution in defining a causal or consequential role of neuroinflammation in AD, but rather focus on identifying its exact pathological contribution.

Similar content being viewed by others
References
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, 5th edn. American Psychiatric Publishing, Arlington
Fratiglioni L, Winblad B, von Strauss E (2007) Prevention of Alzheimer’s disease and dementia. Major findings from the Kungsholmen Project. Physiol Behav 92:98–104
Kinsella K, Velkoff VA (2002) The demographics of aging. Aging Clin Exp Res 14:159–169
Rogers J, Cooper NR, Webster S et al (1992) Complement activation by beta-amyloid in Alzheimer disease. Proc Natl Acad Sci USA 89:10016–10020
McGeer PL, McGeer EG (2013) The amyloid cascade-inflammatory hypothesis of Alzheimer disease: implications for therapy. Acta Neuropathol 126:479–497
Heneka MT, O’banion MK (2007) Inflammatory processes in Alzheimer’s disease. J Neuroimmunol 184:69–91
Damani MR, Zhao L, Fontainhas AM et al (2011) Age-related alterations in the dynamic behavior of microglia. Aging Cell 10:263–276
Sierra A, Gottfried-Blackmore AC, Mc Ewen BS et al (2007) Microglia derived from aging mice exhibit altered inflammatory profile. Glia 55:412–424
Harry GJ (2013) Microglia during development and aging. Pharmacol Ther 139:313–326
Parihar MS, Brewer GJ (2007) Simultaneous age-related depolarization of mitochondrial membrane potential and increased mitochondrial reactive oxygen species production correlate with age-related glutamate excitotoxicity in rat hippocampal neurons. J Neurosci Res 85:1018–1032
Kierdorf K, Prinz M (2013) Factors regulating microglia activation. Front Cell Neurosci 7:44
Czlonkowska A, Kurkowska-Jastrzebska I (2011) Inflammation and gliosis in neurological diseases–clinical implications. J Neuroimmunol 231:78–85
Liu W, Tang Y, Feng J (2011) Cross talk between activation of microglia and astrocytes in pathological conditions in the central nervous system. Life Sci 89:141–146
Cras P, Smith MA, Richey PL et al (1995) Extracellular neurofibrillary tangles reflect neuronal loss and provide further evidence of extensive protein cross-linking in Alzheimer disease. Acta Neuropathol 89:291–295
Seubert P, Vigo-Pelfrey C, Esch F et al (1992) Isolation and quantification of soluble Alzheimer’s beta-peptide from biological fluids. Nature 359:325–327
Sardi F, Fassina L, Venturini L et al (2011) Alzheimer’s disease, autoimmunity and inflammation. The good, the bad and the ugly. Autoimmun Rev 11:149–153
Tejera D, Heneka MT (2016) Microglia in Alzheimer’s disease: the good, the bad and the ugly. Curr Alzheimer Res 13:370–380
Abbott NJ, Patabendige AA, Dolman DE et al (2010) Structure and function of the blood brain barrier. Neurobiol Dis 37:13–25
Crane IJ, Liversidge J (2008) Mechanism of leukocyte migration across the blood–retina-barrier. Semin Immunopathol 30:165–177
Sallusto F, Impellizieri D, Basso C et al (2012) T-cell trafficking in the central nervous system. Immunol Rev 248:216–227
Engelhardt B, Coisne C (2011) Fluids and barriers of the CNS establish immune privilege by confining immune surveillance to a two-walled castle moat surrounding the CNS castle. Fluids Barriers CNS 8:4
Lyck R, Engelhardt B (2012) Going against the tide—how encephalitogenic T cells breach the blood brain barrier. J Vasc Res 49:497–509
Von Andrian UH, Mackay CR (2000) T-cell function and migration. Two sides of the same coin. N Engl J Med 343:1020–1034
Hickey WF, Hsu BL, Kimura H (1991) T lymphocyte entry into the central nervous system. J Neurosci Res 28:254–260
Owens T, Bechmann I, Engelhardt B (2008) Perivascular spaces and the two steps to neuroinflammation. J Neuropathol Exp Neurol 67:1113–1121
Steiner O (2010) Differential roles for endothelial ICAM-1, ICAM-2 and VCAM-1 in shear resistant T cell arrest, polarization, and directed crawling on BBB endothelium. J Immunol 185:4846–4855
Bauer M, Brakebusch C, Coisne C et al (2009) Beta 1 integrins differentially control extravasation of inflammatory cell subsets into the CNS during autoimmunity. Proc Natl Acad Sci USA 106:1920–1925
Shattil SJ, Kim C, Ginsberg MH (2010) The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol 11:288–300
Bullard DC, Hu X, Schoeb TR et al (2007) Intercellular adhesion molecule-1 expression is required on multiple cell types for development of experimental autoimmune encephalomyelitis. J Immunol 178:851–857
Cashman JR, Ghirmai S, Abel KJ et al (2008) Immune defects in Alzheimer’s disease: new medications development. BMC Neurosci 9(Suppl 2):S13
Saresella M, Calabrese E, Marventano I et al (2010) PD1 negative and PD1 positive CD4+ T regulatory cells in mild cognitive impairment and Alzheimer’s disease. J Alzheimer Dis 21:927–938
Wang L, Xie Y, Zhu LJ et al (2010) An Association between immunosenescence and CD4+ CD25+ regulatory T Cells: a systematic review. Biomed Environ Sci 23:327–332
Larbia A, Paweleca G, Witkowskib JM et al (2009) Dramatic shifts in circulating CD4 but not CD8 T cell subsets in mild Alzheimer’s disease. J Alzheimer Dis 17:91–103
Hanke ML, Kielian T (2011) Toll-like receptors in health and disease in the brain: mechanisms and therapeutic potential. Clin Sci 121:367–387
Landreth GE, Reed-Geaghan EG (2009) TLRs in Alzheimer’s disease. Curr Top Microbiol Immunol 336:137–153
Riazi K, Galic MA, Pittman QJ (2010) Contributions of peripheral inflammation to seizure susceptibility: cytokines and brain excitability. Epilepsy Res 89:34–42
Asari Y, Majima M, Sugimoto K et al (1996) Release site of TNF alpha after intravenous and intraperitoneal injection of LPS from Escherichia coli in rats. Shock 5:208–212
Ho Y, Lin Y, Wu C et al (2015) Peripheral inflammation increases seizure susceptibility via the induction of neuroinflammation and oxidative stress in the hippocampus. J Biomed Sci 22:46
Goehler LE, Gaykema RP, Nguyen KT et al (1999) Interleukin-1beta in immune cells of the abdominal vagus nerve: A link between the immune and nervous systems? J Neurosci 19:2799–2806
Nakano Y, Furube E, Morita S et al (2015) Astrocytic TLR4 expression and LPS-induced nuclear translocation of STAT3 in the sensory circumventricular organs of adult mouse brain. J Neuroimmunol 278:144–158
Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8:57–69
Nguyen MD, D’Aigle T, Gowing G et al (2004) Exacerbation of motor neuron disease by chronic stimulation of innate immunity in a mouse model of amyotrophic lateral sclerosis. J Neurosci 24:1340–1349
Chen Z, Jalabi W, Shpargel KB et al (2012) Lipopolysaccharide-induced microglial activation and neuroprotection against experimental brain injury is independent of hematogenous TLR4. J Neurosci 32:11706–11715
Henry CJ, Huang Y, Wynne AM et al (2009) Peripheral lipopolysaccharide challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun 23:309–317
Liu X, Wu Z, Hayashi Y et al (2012) Age-dependent neuroinflammatory responses and deficits in long-term potentiation in the hippocampus during systemic inflammation. Neuroscience 216:133–142
Turola E, Furlan R, Bianco F et al (2012) Microglial Microvesicle Secretion and Intercellular Signaling. Front Physiol 3:149
Rajendran L, Honsho M, Zahn TR et al (2006) Proc Natl Acad Sci USA 2006:11172–11177
Bianco F, Pravettoni E, Colombo A et al (2005) Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol 174:7268–7277
Gonnord P, Delarasse C, Auger R et al (2009) Palmitoylation of the P2X7 receptor, an ATP-gated channel, controls its expression and association with lipid rafts. FASEB J 23:795–805
Antonucci F, Turola E, Riganti L et al (2012) Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J 31:1240
Verderio C, Muzio L, Turola E et al (2012) Myeloid microvesicles are a marker and a therapeutic target for neuroinflammation. Ann Neurol 72:610–624
Joshi P, Turola E, Ruiz A et al (2014) Microglia convert aggregated amyloid-β into neurotoxic forms through the shedding of microvesicles. Cell Death Differ 21:582–593
Lamkanfi M, Dixit VM (2012) Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol 28:137–161
Kayagaki N, Warming S, Lamkanfi M et al (2011) Non-canonical inflammasome activation targets caspase-11. Nature 479:117–121
Martinon F, Burns K, Tschopp J (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 2:417–426
Martinon F, Mayor A, Tschopp J (2009) The inflammasomes: guardians of the body. Annu Rev Immunol 27:229–265
Petrilli V, Papin S, Dostert C et al (2007) Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ 14:1583–1589
Thomas PG, Dash P, Aldridge JR et al (2009) NLRP3 (NALP3/CIAS1/Cryopyrin) mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity 30:566–575
Halle A, Hornung V, Petzold GC et al (2008) The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat Immunol 9:857–865
Masters SL (2012) Specific inflammasomes in complex diseases. Clin Immunol 147:223–228
Heneka MT, Kummer MP, Stutz A et al (2013) NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493:674–678
Marin R (2011) Signalosomes in the brain: relevance in the development of certain neuropathologies such as Alzheimer’s disease. Front Physiol 2:23
Marin R, Guerra B, Alonso R et al (2005) Estrogen activates classical and alternative mechanisms to orchestrate neuroprotection. Curr Neurovasc Res 2:287–301
Guerra B, Diaz M, Alonso R et al (2004) Plasma membrane estrogen receptor mediates neuroprotection against β-amyloid toxicity through activation of Raf1/MEK/ERK cascade in septal-derived cholinergic SN56 cells. J Neurochem 91:99–1099
Ramirez CM, Gonzalez M, Diaz M et al (2009) VDAC and ERα interaction in caveolae from human cortex is altered in Alzheimer’s disease. Mol Cell Neurosci 42:172–183
Amtul Z, Wang L, Westaway D et al (2010) Neuroprotective mechanism conferred by 17beta-estradiol on the biochemical basis of Alzheimer’s disease. Neuroscience 169:781–786
Yue X, Lu M, Lancaster T et al (2005) Brain estrogen deficiency accelerates A(beta) plaque formation in an Alzheimer’s disease animal model. Proc Natl Acad Sci USA 102:19198–19203
Alvarez-De-La-Rosa M, Silva I, Nilsen J et al (2005) Estradiol prevents neural tau hyperphosphorylation characteristic of Alzheimer’s disease. Ann NY Acad Sci 1052:210–224
Pike CJ, Carroll JC, Rosario ER et al (2009) Protective actions of sex steroid hormones in Alzheimer’s disease. Front Neuroendocrinol 30:239–258
Simpkins JW, Yi KD, Yang SH et al (2010) Mitochondrial mechanisms of estrogen neuroprotection. Biochim Biophys Acta 1800:1113–1120
Pike CJ (1999) Estrogen modulates neuronal Bcl-xL expression and β-amyloid-induced apoptosis: relevance to Alzheimer’s disease. J Neurochem 72:1552–1563
Nilsen J (2008) Estradiol and neurodegenerative oxidative stress. Front Neuroendocrinol 29:463–475
Borras C, Sastre J, Garcia-Sala D et al (2003) Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic Biol Med 34:546–552
Barha CK, Galea LA (2010) Influence of different estrogens on neuroplasticity and cognition in the hippocampus. Biochim Biophys Acta 1800:1056–1067
Foy MR, Baudry M, Diaz Brinton R et al (2008) Estrogen and hippocampal plasticity in rodent models. J Alzheimers Dis 15:589–603
Goodman Y, Bruce AJ, Cheng B et al (1996) Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid β-peptide toxicity in hippocampal neurons. J Neurochem 66:1836–1844
Morrison JH, Baxter MG (2012) The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev Neurosci 13:240–250
Nilsen J, Irwin RW, Gallaher TK et al (2007) Estradiol in vivo regulation of brain mitochondrial proteome. J Neurosci 27:14069–14077
Asthana S, Baker LD, Craft S et al (2001) High-dose estradiol improves cognition for women with AD: results of a randomized study. Neurology 57:605–612
Schneider LS, Farlow MR, Henderson VW et al (1996) Effects of estrogen replacement therapy on response to tacrine in patients with Alzheimer’s disease. Neurology 46:1580–1584
D’Andrea MR (2005) Add Alzheimer’s disease to the list of autoimmune diseases. Med Hypotheses 64:458–463
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all Authors, the corresponding author states that there is no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Guerriero, F., Sgarlata, C., Francis, M. et al. Neuroinflammation, immune system and Alzheimer disease: searching for the missing link. Aging Clin Exp Res 29, 821–831 (2017). https://doi.org/10.1007/s40520-016-0637-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-016-0637-z