Abstract
Background
Gold-standard psychological and pharmacological treatments for bulimic-spectrum eating disorders only result in remission for around 50% of patients; patients with affective lability and impulsivity represent a subgroup with particularly poor outcomes. Both dialectical behavior therapy (DBT), a treatment for emotion dysregulation, and lamotrigine, a mood stabilizer, have demonstrated promise for targeting affective lability and impulsivity; however, data exploring the combination of these interventions remain limited.
Objective
We followed a group of women with recurrent dysregulated eating behaviors (N = 62) throughout intensive DBT treatment and compared the symptom trajectory of those prescribed lamotrigine (n = 28) and those who were not (n = 34).
Method
Participants completed surveys every 2 weeks throughout treatment.
Results
Group analyses suggested that all participants self-reported decreases in emotional reactivity, negative urgency, and symptoms of borderline personality disorder (BPD). The lamotrigine group reported greater elevations in BPD symptoms at baseline, but demonstrated steeper decreases in emotion and behavioral dysregulation than the non-matched comparison group. Within-subject analyses suggested that within the lamotrigine group, subjects reported greater decreases in symptoms following prescription of lamotrigine.
Conclusions
Findings provide initial data suggesting that lamotrigine could be useful as an adjunctive treatment for patients with affective lability and impulsivity.
Level of evidence
IV, time series without randomization.
Similar content being viewed by others
Introduction
Despite progress in our understanding of the etiology and treatment of eating disorders (EDs), a significant number of patients do not achieve lasting remission of symptoms [1]. Individuals with mood lability and impulsive behaviors represent one group with a consistently poorer response to existing ED treatments [2]. For example, 25% or more of individuals with bulimia nervosa have “multi-impulsive” behaviors and are particularly prone to a chronic course of illness and high mortality. This subgroup of ED patients are defined by dysregulated eating behaviors (e.g., binge eating; self-induced vomiting) and co-occurring disinhibited behaviors and typically have high rates of psychiatric comorbidities, such as self-harm and substance use disorders [3, 4]. Consistent data suggest that impulsivity (e.g., self-injury) relates to drop-out from evidence-based treatments [5,6,7]. Further, patents with EDs who endorse characteristics of Cluster B personality disorders and bipolarity, including affective, identity, and behavioral instability, have worse outcomes [8,9,10]. Evaluation of interventions that may be effective for this specific subgroup of adults is essential to reducing the negative outcomes associated with these illnesses.
One promising treatment for multi-impulsive patients with EDs and high affective lability is dialectical behavior therapy (DBT). DBT was originally developed to target recurrent suicidal and self-harm behaviors in women with borderline personality disorder (BPD) and has been studied extensively as a treatment for behaviors associated with emotion regulation problems [11]. The DBT model teaches individuals coping strategies to facilitate effective emotional regulation [12]. DBT is considered the first-line treatment for emotion dysregulation in BPD and suicidality, and preliminary data from small-scale, open trials indicate that DBT may be effective for emotionally dysregulated ED patients [13, 14].
Though the content of DBT is well suited to the symptoms of chronic, multi-impulsive EDs, elevations in mood lability and a tendency toward rash action in the context of mood lability could interfere with engaging in treatment and learning and applying DBT skills. Indeed, data suggest that this patient population struggles to engage in other evidence-based treatments (e.g., cognitive-behavioral therapy) [15]. As such, medications that stabilize mood and reduce impulse control could serve as useful adjunct to DBT and other concurrent psychotropic medications, such as antidepressant medications. Specifically, mood stabilizers may allow dysregulated patients to better acquire and generalize skills taught in DBT. The antiepileptic drug lamotrigine, which is widely used as a mood stabilizer in bipolar disorder, has demonstrated initial success in treating patient populations characterized by labile moods and impulsivity [16, 17], although recent results from well-powered randomized controlled trials in BPD suggest it may be less efficacious [18]. With regard to its effectiveness for treating EDs, an initial case series of five patients within our program indicated that concurrent lamotrigine, often alongside an antidepressant medication, and DBT were associated with decreases in mood reactivity, impulsive behaviors, and ED behavior [19]. Results from a second open trial revealed significant decreases in impulsive and dysregulated behaviors when lamotrigine was added to DBT and concurrent selective-serotonin reuptake inhibitors (SSRIs) for nine women with EDs [20]. However, given that these investigations evaluated the simultaneous use of DBT and lamotrigine, it is unclear whether lamotrigine provides additive benefit over and above DBT.
The current study aimed to build upon existing case series research by including a comparison group of patients from the same cohort. Specifically, we compared clinical outcomes in two groups of patients with bulimic-spectrum EDs in a DBT-based program: patients who received concurrent lamotrigine treatment, and patients who were not prescribed lamotrigine. Given past work in this domain, we predicted that the addition of lamotrigine would be associated with a greater decline in affective lability and behavioral impulsivity.
Methods
Participants
Participants were patients enrolled in a partial hospitalization program/intensive outpatient program for EDs. To be considered for inclusion in the study, participants were required to report dysregulated eating behaviors (loss-of-control eating episodes of any size and/or compensatory behaviors) at least once per week for the past 3 months. Participants were also required to be 18–45 years old, to be female, to be medically stable, and weigh between 75 and 135% of the expected weight for their height based on the Metropolitan Life Insurance tables [21]. Patients with bipolar-spectrum, schizophrenia-spectrum, or other psychotic disorders were excluded. There were no eligibility criteria related to other medications prescribed to participants in the study.
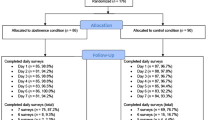
Sixty-two patients met initial inclusion criteria for the study and were enrolled in study procedures. Over the course of treatment, 45.16% of the sample (n = 28) was prescribed lamotrigine, and 54.84% (n = 34) served as the non-matched comparison group. All participants provided written informed consent, and the local institutional review board approved this study.
Measures
Clinical interviews
We used the Structured Clinical Interview for DSM-5: Eating Disorders Module [22] to assess current ED diagnoses in our sample, and the MINI International Neuropsychiatric Interview [23] to assess comorbid psychiatric diagnoses. For a detailed description of the interviews, please see the Supplement.
Biweekly self-report measurements
Participants completed a range of well-validated self-report measurements every 2 weeks over the course of treatment, including the Borderline Evaluation of Severity Over Time (BEST) [24], the Emotional Reactivity Scale (ERS) [25], the UPPS-P Negative Urgency Scale (UPPS-P) [26], and single item measures from the Eating Disorders Examination—Questionnaire (EDE-Q; [27]) to measure binge/purge frequency. For a detailed description of these biweekly assessments, please see the Supplement.
Treatments of interest
Dialectical behavior therapy
Each patient was enrolled in an ED partial hospitalization and intensive outpatient program. The program operates using full-model DBT and therefore includes individual sessions, skills groups, therapist consultation, and phone coaching, as outlined by Linehan [12] with adaptations described elsewhere [28]. The DBT program is administered in a partial hospitalization setting, and patients are admitted to the program if they endorse clinically significant ED symptoms that warrant admission to a higher level of care or have experienced treatment failure at a lower level of care. As described elsewhere [14, 29], ED symptoms can be incorporated readily into the DBT framework; within the current program, there were no major elements of the traditional DBT framework that were omitted, and the unique ED-specific additions to standard DBT included regular meetings with a dietician, additional groups with content from other cognitive-behavioral therapies for EDs, and medication management. Weekly individual therapy followed a DBT framework. All patients enrolled in the program begin by attending treatment for 10 h a day, 6 days a week. Pending treatment progress, patients are stepped down to 6 h a day, 5–6 days a week, followed by intensive outpatient programming (4 h a day; 3–5 days a week).
Lamotrigine
Participants were prescribed lamotrigine by one of two staff psychiatrists or one of two psychiatric nurse practitioners. Consistent with recommendations for use of SSRIs for the treatment of both BN symptoms and co-occurring mood symptoms in EDs [30, 31], the majority of patients were also prescribed an SSRI. For lamotrigine, dosing followed a more conservative schedule than that recommended by the FDA for bipolar disorder. Specifically, study providers initiated lamotrigine dosages at 25 mg/day for ≥ 2 weeks, then increased to 50 mg/day for ≥ 2 weeks, then generally increased the dose at a rate no faster than 25 mg/day every 1–2 weeks, contingent on patient response to the medication. As such, the titration schedule varied across participants and was based on patient response, as well as staff psychiatrists’ clinical assessment. Therapeutic doses for the group ranged between 75 and 400 mg/day of lamotrigine.
Procedures
Patients who met initial eligibility criteria were provided with information regarding the study within 1 week of partial hospitalization program (PHP) admission. Semi-structured interviews were conducted by bachelor’s- or doctoral-level research staff who were supervised weekly by two psychologists with extensive training in the administration of diagnostic interviews and attended weekly consensus meetings. Eligible participants completed biweekly assessments throughout treatment. Medication information related to changes in lamotrigine or other psychotropic medications was gathered weekly from the patient’s chart.
Statistical analysis
We classified patients into groups based on whether they were prescribed and took lamotrigine during treatment. Initial analyses compared groups on diagnostic and self-reported variables to test their equivalence.
We examined the effect of lamotrigine on symptoms of BPD, negative urgency, emotional reactivity, binge eating, and purging using two approaches. First, we conducted multilevel models (MLMs) using the full sample to test for group x time interaction effects on symptoms. Because participants were started on lamotrigine at different points in treatment (range = 0–126 days following admission to PHP), we centered time for the lamotrigine group around the initial prescription date. “Day 0” for the non-matched comparison group was the date of admission to the clinic.
Our second set of analyses examined symptom change over time within the lamotrigine group (n = 28), excluding comparison subjects. We conducted a series of piecewise MLMs to explore changes in symptoms pre- and post-lamotrigine initiation (i.e., first day taking lamotrigine). For these analyses, time was modeled continuously and centered around the time at which participants were prescribed lamotrigine.
All MLMs were conducted using the R package lme4 [31]. Models were fitted in a stepwise fashion, and all models reported in the current investigation represent random intercept, fixed slope models. MLMs account for data that are nested (i.e., multiple observations over time). MLMs estimate both fixed or average effects across a sample, and random effects, which model variability in effects across subjects or time points. In the current investigation, we report fixed effects, or the average effect of each variable in the model across the full sample. To handle missing data, we used full-information maximum likelihood estimation. For binge eating and purging analyses (i.e., count data), we used a negative binomial distribution, in accordance with recommendations [29].
Results
Baseline differences between groups
One participant provided informed consent but then dropped out of treatment during the interviewing procedures; this participant is not included in the final total for the sample and in the Tables. Frequencies of ED diagnoses, co-occurring diagnoses, and prescribed medications at baseline are available in Table 1, as well as means and standard deviations for outcome variables. These counts include all subjects consented into the study other than the participant who did not complete the interviewing procedures. On average, participants met criteria for 2–3 co-occurring diagnoses. The average length of ED illness was 11.65 years (SD = 7.69 years).
As participants were not prospectively randomized to treatment groups, we conducted two series of comparisons across treatment groups. Comparison of mean differences across groups in variables collected at intake to treatment indicate significant differences in several domains, including emotion reactivity, negative urgency, and symptoms of borderline personality disorder, such that individuals prescribed lamotrigine had higher scores in all domains (Table 1). In addition to exploring baseline differences across groups, we compared baseline assessment of the non-matched comparison group with assessments taken directly before prescription of lamotrigine in the medication group, as these time points reflected our operationalization of “baseline,” in longitudinal analyses. Results from these comparisons reveal smaller group differences only in BEST scores.
Longitudinal group differences
The number of assessments completed by participants ranged from 0 to 20 (mean = 7.13; SD = 3.71). In total, seven participants left treatment against clinical advice prior to discharge, and 15 biweekly assessments (3.39% of total assessments) were missing due to patient absence from the program or declination to complete. We used all available data within each model, including the data of those participants with missing time points and/or those who dropped out of treatment.
Results from each MLM are presented in Table 2 and in Fig. 1. All models indicated statistically significant main effects of time, suggesting that on average, all patients showed improvements on all measures over the course of treatment. Additional main effects and interactions are reported below.
BPD symptoms
Results indicated a significant effect of group and a significant interaction between time and group on BEST scores. Inspection of these effects suggested that the lamotrigine group demonstrated average BEST scores that were consistently higher than those of the non-matched comparison group, but that the lamotrigine group demonstrated steeper mean decreases in BEST scores over time compared to non-matched comparison patients (Fig. 1).
Negative urgency
We detected a significant group x time interaction on UPPS-P scores. The directionality of these effects suggested that on average, all participants self-reported decreases in negative urgency over the course of treatment, and the slope of this effect was greater among the non-matched comparison group.
Emotional reactivity, DBT skills use, and bulimic symptoms
Other than time, no effects on ERS scores, WCCL scores, binge eating, or purging were statistically significant.
Within-group analyses (Table 3; Fig. 2)
BEST scores decreased significantly both before and after lamotrigine initiation. This effect increased in size following the prescription of lamotrigine. Binge eating, purging, and scores on the UPPS-P, ERS, and WCCL all demonstrated a similar pattern; however, for these measures, there was no significant change in symptoms or change in DBT skills use before lamotrigine, but a significant decrease in symptoms and increase in DBT skills use after lamotrigine.
Discussion
ED patients with multi-impulsive behaviors and BPD features such as affective lability often show poor treatment response and high rates of relapse [10]. The current study tested a mood stabilizer, lamotrigine, as an adjunctive intervention to DBT for this treatment-refractory population. This is the first study to build on an initial case series and open trial of lamotrigine for EDs by including a non-matched comparison group. Despite many limitations of the quasi-experimental study design, results provide some support for the usefulness of lamotrigine in combination with DBT for the treatment of ED patients with high affective lability and behavioral impulsivity.
All patients reported decreases over time in symptoms of BPD, affective lability, negative urgency, binge eating, and purging, and increases in adaptive skill use. However, patients prescribed lamotrigine showed a steeper decrease in BPD symptoms than those who did not. While baseline differences between groups on BPD symptoms make this interaction effect challenging to interpret definitively, and it is possible these differences simply represent regression to the mean, our findings are broadly consistent with observations that lamotrigine is helpful in decreasing impulsive behaviors associated with BPD [17, 32]. The lack of effect of lamotrigine on affective lability as operationalized by the ERS is inconsistent with several studies suggesting that lamotrigine could influence the intensity and lability of emotions in populations with BPD [17, 33], although null results for the medication’s effectiveness in targeting affective lability are not unprecedented [18]. Further, our results suggested greater decreases in negative urgency in the non-matched comparison group compared to the lamotrigine group. However, visual inspection of these differences (Fig. 1) suggested this difference was slight in nature.
Within the subgroup of patients who received lamotrigine, emotional reactivity, negative urgency, binge eating, purging, and skills use changed at a significant rate only after the initiation of lamotrigine. These analyses are limited by the nature of the data collection procedures (e.g., variability in the time frame before and after prescription); however, they do provide some preliminary evidence that that in a group of patients who reports significant affective lability and impulsivity, prescription of lamotrigine is associated with a significant decrease in self-reported emotional reactivity and impulsivity. Of note, these patients did not report significant changes in skills use, emotional reactivity, and impulsivity prior to taking lamotrigine despite being enrolled in the DBT program, consistent with the clinical prediction that lamotrigine may enable patients to better engage with and benefit from the treatment [19, 20].
The mechanism of action of lamotrigine is not yet clear; however, data from functional neuroimaging studies suggest that it primarily targets prefrontal cortical areas integral to cognitive and behavioral control. Acute doses increase resting-state connectivity among prefrontal regions [34], and after 12 weeks, lamotrigine increases activation in prefrontal regions when individuals with bipolar disorder view sad faces [35]. Longitudinal neuroimaging research paired with randomized, controlled trials are needed; however, existing findings suggest that lamotrigine’s therapeutic effects in our population may have been related to increases in prefrontal activation and connectivity that could improve the ability to control one’s behavior.
Notably, our findings are somewhat inconsistent with a recent large, randomized trial of lamotrigine for the treatment of BPD, which suggested no significant benefits of lamotrigine for BPD or depressive symptoms [18]. There are several potential explanations for the inconsistency. It is certainly possible that the methodological limitations of our study (e.g., lack of randomization; differences in groups)—detailed below—can account for our observed effects. It is also possible that other methodological differences across these studies accounts for the inconsistency. For instance, although participants in the Crawford et al. study [18] were able to enroll in other types of treatment during the trial, the rates of psychotherapy use and receipt of evidence-based therapy were not reported; therefore, it is possible that positive effects of lamotrigine only occur when administered in conjunction with evidence-based psychological treatments for BPD. Further, Crawford et al. [18] used different outcome measurements than we employed in our study and recruited individuals with complex, full-threshold BPD, limiting our ability to make direct comparisons. Altogether, while it is challenging to draw parallels between our results and that of the recent randomized controlled trial in BPD [18], the null results that emerged in this prior trial highlight the need for future rigorous attempts to replicate our findings in a large sample of individuals with dysregulated eating behaviors.
Several study limitations should be acknowledged. First, many of the patients included in the study were prescribed various medications throughout their treatment stay in addition to lamotrigine, and this makes it difficult to isolate which medications may be contributing to symptom change. However, our psychiatric providers often follow some common prescribing patterns, particularly regarding concurrent antidepressant use. As shown in Table 1, comorbid depressive and anxiety disorders are extremely common in these patients. For this reason, most patients are continued on or started on an antidepressant at the time of admission and remain on the antidepressant throughout treatment. In patients with bulimic-spectrum disorders, it is common to titrate to high-dose selective-serotonin reuptake inhibitors [30, 31], so an initial step is often dose maximization of an antidepressant. If this does not significantly improve all target symptoms, lamotrigine may be added. For patients who enter the program already having limited response to high-dose antidepressants, lamotrigine may be added at or shortly after admission.
Second, the nature of the population presented some challenges for self-report data collection. Given participants’ emotional lability, self-report data may reflect mood in any given moment and may not be an accurate depiction of the mean or variance of an individual’s mood state over a 2-week period. Furthermore, the affective instability and impulsivity of the group presented challenges to consistent data collection, as participants intermittently declined to complete assessments or took unexcused absences from treatment, where assessments were administered. Third, as noted previously, there was considerable variance in the initiation timing and dosing trajectory of lamotrigine across individuals in the study, as well as variance in the length of treatment, which makes the trajectory of treatment outcome scores difficult to interpret. Related to this limitation, we did not assess plasma or serum concentrations of lamotrigine and thus only measured adherence using patient self-report. Fourth, group assignment was not randomized, which perhaps represents the greatest limitation to our study. While our findings cannot be taken as definitive evidence of beneficial effects of lamotrigine in this population, the inclusion of a non-matched comparison group builds on promising initial evidence from open trials, supporting the assertion that future rigorous work in this domain is warranted.
The current study also had various strengths. First, relative to existing treatment studies of this severely dysregulated population, the current study includes a large sample size. Second, although a small group of patients had short treatment stays after violating the terms of behavioral contracts in treatment or discharging to higher levels of care, the mean length of stay across both groups was 72 days. The extended nature of treatment and frequent assessments provided us with numerous data points for most participants and a comprehensive picture of symptoms over the course of treatment. Third, the use of semi-structured interviews ensures accurate diagnoses across all individuals in the sample.
Our findings from a quasi-experimental design provide a number of important avenues for future research. Results support larger, randomized controlled trials to examine the effects of lamotrigine with and without DBT in patients with EDs and severe affective and behavioral dysregulation. Investigation of lamotrigine’s effects using alternative measurements, such as behavioral tasks, would also represent an important future direction. Additionally, future studies should explore whether the combination of lamotrigine with DBT is more effective in treating ED patients with full-threshold BPD.
Conclusions
Considering data suggesting poor outcomes for individuals who struggle with chronic, impulsive ED symptoms and affective dysregulation, identifying potential treatments that can serve as an adjunct or alternative to existing interventions is a critical endeavor. In the current study, we explored the effect of lamotrigine as an adjunctive treatment to intensive DBT for EDs in individuals who struggled with impulsivity and affective lability. Despite limitations of the study design, our results provide tentative support for the use of lamotrigine in patients with binge-purge EDs, concurrent BPD features, and high levels of emotion dysregulation. Moving forward, future work should make use of randomized designs and matched control groups to pursue more definitive tests of lamotrigine as a promising treatment.
Strength and limits
Limitations of the study include lack of randomization, resulting in unequal groups, as well as the naturalistic design of the study. Strengths include the focus on a generally understudied group in ED treatment research, the longitudinal design, and a relatively large sample compared to other work in this domain.
What is already known on this subject?
Currently, there are very few effective treatments for individuals with multi-impulsive, bulimic-spectrum disorders, particularly those who have experienced prior treatment failure. Several case series have indicated some effectiveness for lamotrigine in the treatment of bulimic-spectrum disorders.
What this study adds?
This study provides initial pilot support for the fact that the prescription of lamotrigine is associated with clinical benefit; randomized controlled trials represent the next step in this line of work.
Availability of data and material
The data supporting this investigation will be made available upon reasonable request to the corresponding author.
Code availability
The code supporting the analyses in the current manuscript will be made available upon reasonable request to the corresponding author.
References
Keel PK, Brown TA (2010) Update on course and outcome in eating disorders. Int J Eat Disord 43:195–204. https://doi.org/10.1002/eat.20810
Fichter MM, Quadflieg N, Rief W (1994) Course of multi-impulsive bulimia. Psychol Med 24:591–604. https://doi.org/10.1017/s0033291700027744
Corstorphine E, Waller G, Lawson R, Ganis C (2007) Trauma and multi-impulsivity in the eating disorders. Eat Behav 8:23–30. https://doi.org/10.1016/j.eatbeh.2004.08.009
Svirko E, Hawton K (2007) Self-injurious behavior and eating disorders: the extent and nature of the association. Suicide Life Threat Behav 37:409–421. https://doi.org/10.1521/suli.2007.37.4.409
Agras WS, Crow SJ, Halmi KA, Mitchell JE, Wilson GT, Kraemer HC (2000) Outcome predictors for the cognitive behavior treatment of bulimia nervosa: data from a multisite study. Am J Psychiatry 157:1302–1308. https://doi.org/10.1176/appi.ajp.157.8.1302
Favaro A, Santonastaso P (2000) Self-injurious behavior in anorexia nervosa. J Nerv Ment Dis 188:537–542. https://doi.org/10.1097/00005053-200008000-00010
Peake KJ, Limbert C, Whitehead L (2005) Gone, but not forgotten: an examination of the factors associated with dropping out from treatment of eating disorders. Eur Eat Disord Rev 13:330–337. https://doi.org/10.1002/erv.645
Helverskov JL, Clausen L, Mors O, Frydenberg M, Thomsen PH, Rokkedal K (2010) Trans-diagnostic outcome of eating disorders: a 30-month follow-up study of 629 patients. Eur Eat Disord Rev 18:453–463. https://doi.org/10.1002/erv.1025
Rossiter EM, Agras WS, Telch CF, Schneider JA (1993) Cluster B personality disorder characteristics predict outcome in the treatment of bulimia nervosa. Int J Eat Disord 13:349–357. https://doi.org/10.1002/1098-108X(199305)13:4%3c349::AID-EAT2260130403%3e3.0.CO;2-C
Vall E, Wade TD (2015) Predictors of treatment outcome in individuals with eating disorders: a systematic review and meta-analysis. Int J Eat Disord 48:946–971. https://doi.org/10.1002/eat.22411
Kliem S, Kröger C, Kosfelder J (2010) Dialectical behavior therapy for borderline personality disorder: a meta-analysis using mixed-effects modeling. J Consult Clin Psychol 78:936–951. https://doi.org/10.1037/a0021015
Linehan MM (2018) Cognitive-behavioral treatment of borderline personality disorder. Guilford Publications
Federici A, Wisniewski L, Ben-Porath D (2012) Description of an intensive dialectical behavior therapy program for multidiagnostic clients with eating disorders. J Couns Dev 90:330–338. https://doi.org/10.1002/j.1556-6676.2012.00041.x
Wisniewski L, Kelly E (2003) The application of dialectical behavior therapy to the treatment of eating disorders. Cogn Behav Pract 10:131–138. https://doi.org/10.1016/S1077-7229(03)80021-4
Coker S, Vize C, Wade T, Cooper PJ (1993) Patients with bulimia nervosa who fail to engage in cognitive behavior therapy. Int J Eat Disord 13:35–40. https://doi.org/10.1002/1098-108x(199301)13:1%3c35::aid-eat2260130105%3e3.0.co;2-n
Preston GA, Marchant BK, Reimherr FW, Strong RE, Hedges DW (2004) Borderline personality disorder in patients with bipolar disorder and response to lamotrigine. J Affect Disord 79:297–303. https://doi.org/10.1016/S0165-0327(02)00358-0
Reich DB, Zanarini MC, Bieri KA (2009) A preliminary study of lamotrigine in the treatment of affective instability in borderline personality disorder. Int Clin Psychopharmacol 24:270–275. https://doi.org/10.1097/YIC.0b013e32832d6c2f
Crawford MJ, Sanatinia R, Barrett B, Cunningham G, Dale O, Ganguli P, Lawrence-Smith G, Leeson VC, Lemonsky F, Lykomitrou-Matthews G, Montgomery A, Morriss R, Munjiza J, Paton C, Skorodzien I, Singh V, Tan W, Tyrer P, Reilly JG (2018) Lamotrigine for people with borderline personality disorder: a RCT. Health Technol Assess Winch Engl 22:1–68. https://doi.org/10.3310/hta22170
Trunko ME, Schwartz TA, Marzola E, Klein AS, Kaye WH (2014) Lamotrigine use in patients with binge eating and purging, significant affect dysregulation, and poor impulse control. Int J Eat Disord 47:329–334. https://doi.org/10.1002/eat.22234
Trunko ME, Schwartz TA, Berner LA, Cusack A, Nakamura T, Bailer UF, Chen JY, Kaye WH (2017) A pilot open series of lamotrigine in DBT-treated eating disorders characterized by significant affective dysregulation and poor impulse control. Borderline Pers Disord Emot Dysregul 4:21. https://doi.org/10.1186/s40479-017-0072-6
Metropolitan Life Insurance Company (1957) New weight standards for men and women. Stat Bull 40:1–4
First M, Williams J, Karg R, Spitzer R (2015) Structured clinical interview for DSM-5—research version (SCID-5 for DSM-5, research version; SCID-5-RV). American Psychiatric Association, Arlington
Sheehan D, Lecrubier Y, Harnett Sheehan K, Janavs J, Weiller E, Keskiner A, Schinka J, Knapp E, Sheehan M, Dunbar G (1997) The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. Eur Psychiatry 12:232–241. https://doi.org/10.1016/S0924-9338(97)83297-X
Pfohl B, Blum N, St. John D, McCormick B, Allen J, Black DW (2009) Reliability and validity of the borderline evaluation of severity over time (BEST): a self-rated scale to measure severity and change in persons with borderline personality disorder. J Personal Disord 23:281–293. https://doi.org/10.1521/pedi.2009.23.3.281
Nock MK, Wedig MM, Holmberg EB, Hooley JM (2008) The emotion reactivity scale: development, evaluation, and relation to self-injurious thoughts and behaviors. Behav Ther 39:107–116. https://doi.org/10.1016/j.beth.2007.05.005
Lynam DR, Smith GT, Whiteside SA, Cynders MA (2006) The UPPS-P: assessing five personality pathways to impulsive behavior. West Lafayette Purdue Univ 10
Fairburn CG (2008) Cognitive behavior therapy and eating disorders. Guilford Press
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Schaumberg K, Reilly EE, Anderson LM, Gorrell S, Wang SB, Sala M (2018) Improving prediction of eating-related behavioral outcomes with zero-sensitive regression models. Appetite 129:252–261. https://doi.org/10.1016/j.appet.2018.06.030
Bacaltchuk J, Hay P, Mari JJ (2000) Antidepressants versus placebo for the treatment of bulimia nervosa: a systematic review. Aust NZ J Psychiatry 34:310–317. https://doi.org/10.1080/j.1440-1614.2000.00709.x
Bacaltchuk J, Hay PP (2003) Antidepressants versus placebo for people with bulimia nervosa. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003391
Akingbala F, Dhanani N, Sherwood Brown E (2006) Impulsivity in patients with bipolar disorder and cocaine or amphetamine dependence given lamotrigine. J Dual Diagn 2:73–83. https://doi.org/10.1300/J374v02n03_07
Tritt K, Nickel C, Lahmann C, Leiberich PK, Rother WK, Loew TH, Nickel MK (2005) Lamotrigine treatment of aggression in female borderline-patients: a randomized, double-blind, placebo-controlled study. J Psychopharmacol (Oxf) 19:287–291. https://doi.org/10.1177/0269881105051540
Li X, Large CH, Ricci R, Taylor JJ, Nahas Z, Bohning DE, Morgan P, George MS (2011) Using interleaved transcranial magnetic stimulation/functional magnetic resonance imaging (fMRI) and dynamic causal modeling to understand the discrete circuit specific changes of medications: lamotrigine and valproic acid changes in motor or prefrontal effective connectivity. Psychiatry Res Neuroimaging 194:141–148. https://doi.org/10.1016/j.pscychresns.2011.04.012
Jogia J, Haldane M, Cobb A, Kumari V, Frangou S (2008) Pilot investigation of the changes in cortical activation during facial affect recognition with lamotrigine monotherapy in bipolar disorder. Br J Psychiatry 192:197–201. https://doi.org/10.1192/bjp.bp.107.037960
Funding
This work was supported by the Hilda and Preston Davis Foundation. Dr. Berner was supported by Grants from the National Institutes of Mental Health (F32MH108311; K23MH118418).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Ethics approval
The investigation was approved by the University of California, San Diego Institutional Review Board.
Consent to participate/for publication
All subjects provided consent to participate in the study and to have their data published in aggregate form.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reilly, E.E., Berner, L.A., Trunko, M.E. et al. Evaluating the use of lamotrigine to reduce mood lability and impulsive behaviors in adults with chronic and severe eating disorders. Eat Weight Disord 27, 1775–1785 (2022). https://doi.org/10.1007/s40519-021-01320-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40519-021-01320-3