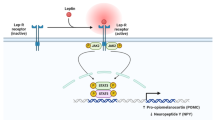
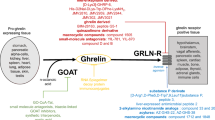
Abstract
Ghrelin is a gastric hormone circulating in acylated (AG) and unacylated (UnAG) forms. This narrative review aims at presenting current emerging knowledge on the impact of ghrelin forms on energy balance and metabolism. AG represents ~ 10% of total plasma ghrelin, has an appetite-stimulating effect and is the only form for which a receptor has been identified. Moreover, other metabolic AG-induced effects have been reported, including the modulation of glucose homeostasis with stimulation of liver gluconeogenesis, the increase of fat mass and the improvement of skeletal muscle mitochondrial function. On the other hand, UnAG has no orexigenic effects, however recent reports have shown that it is directly involved in the modulation of skeletal muscle energy metabolism by improving a cluster of interlinked functions including mitochondrial redox activities, tissue inflammation and insulin signalling and action. These findings are in agreement with human studies which show that UnAG circulating levels are positively associated with insulin sensitivity both in metabolic syndrome patients and in a large cohort from the general population. Moreover, ghrelin acylation is regulated by a nutrient sensor mechanism, specifically set on fatty acids availability. These recent findings consistently point towards a novel independent role of UnAG as a regulator of muscle metabolic pathways maintaining energy status and tissue anabolism. While a specific receptor for UnAG still needs to be identified, recent evidence strongly supports the hypothesis that the modulation of ghrelin-related molecular pathways, including those involved in its acylation, may be a potential novel target in the treatment of metabolic derangements in disease states characterized by metabolic and nutritional complications.
Level of evidence Level V, narrative review.
Similar content being viewed by others
References
Murphy KG, Bloom SR (2006) Gut hormones and the regulation of energy homeostasis. Nature 444(7121):854–859. https://doi.org/10.1038/nature05484
Badman MK, Flier JS (2005) The gut and energy balance: visceral allies in the obesity wars. Science 307(5717):1909–1914. https://doi.org/10.1126/science.1109951
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402(6762):656–660. https://doi.org/10.1038/45230
Inui A, Asakawa A, Bowers CY, Mantovani G, Laviano A, Meguid MM, Fujimiya M (2004) Ghrelin, appetite, and gastric motility: the emerging role of the stomach as an endocrine organ. FASEB J 18(3):439–456. https://doi.org/10.1096/fj.03-0641rev
Bowers CY, Momany F, Reynolds GA, Chang D, Hong A, Chang K (1980) Structure–activity relationships of a synthetic pentapeptide that specifically releases growth hormone in vitro. Endocrinology 106(3):663–667. https://doi.org/10.1210/endo-106-3-663
Bowers CY, Momany FA, Reynolds GA, Hong A (1984) On the in vitro and in vivo activity of a new synthetic hexapeptide that acts on the pituitary to specifically release growth hormone. Endocrinology 114(5):1537–1545. https://doi.org/10.1210/endo-114-5-1537
Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, Paress PS, Diaz C, Chou M, Liu KK, McKee KK, Pong SS, Chaung LY, Elbrecht A, Dashkevicz M, Heavens R, Rigby M, Sirinathsinghji DJ, Dean DC, Melillo DG, Patchett AA, Nargund R, Griffin PR, DeMartino JA, Gupta SK, Schaeffer JM, Smith RG, Van der Ploeg LH (1996) A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 273(5277):974–977
Kojima M, Kangawa K (2005) Ghrelin: structure and function. Physiol Rev 85(2):495–522. https://doi.org/10.1152/physrev.00012.2004
Tomasetto C, Karam SM, Ribieras S, Masson R, Lefebvre O, Staub A, Alexander G, Chenard MP, Rio MC (2000) Identification and characterization of a novel gastric peptide hormone: the motilin-related peptide. Gastroenterology 119(2):395–405
Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K (2001) Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab 86(10):4753–4758. https://doi.org/10.1210/jcem.86.10.7885
Sakata I, Nakamura K, Yamazaki M, Matsubara M, Hayashi Y, Kangawa K, Sakai T (2002) Ghrelin-producing cells exist as two types of cells, closed- and opened-type cells, in the rat gastrointestinal tract. Peptides 23(3):531–536
Knerr I, Herzog D, Rauh M, Rascher W, Horbach T (2006) Leptin and ghrelin expression in adipose tissues and serum levels in gastric banding patients. Eur J Clin Investig 36(6):389–394. https://doi.org/10.1111/j.1365-2362.2006.01642.x
Korbonits M, Bustin SA, Kojima M, Jordan S, Adams EF, Lowe DG, Kangawa K, Grossman AB (2001) The expression of the growth hormone secretagogue receptor ligand ghrelin in normal and abnormal human pituitary and other neuroendocrine tumors. J Clin Endocrinol Metab 86(2):881–887. https://doi.org/10.1210/jcem.86.2.7190
Volante M, Allia E, Gugliotta P, Funaro A, Broglio F, Deghenghi R, Muccioli G, Ghigo E, Papotti M (2002) Expression of ghrelin and of the GH secretagogue receptor by pancreatic islet cells and related endocrine tumors. J Clin Endocrinol Metab 87(3):1300–1308. https://doi.org/10.1210/jcem.87.3.8279
Wierup N, Svensson H, Mulder H, Sundler F (2002) The ghrelin cell: a novel developmentally regulated islet cell in the human pancreas. Regul Pept 107(1–3):63–69
Mori K, Yoshimoto A, Takaya K, Hosoda K, Ariyasu H, Yahata K, Mukoyama M, Sugawara A, Hosoda H, Kojima M, Kangawa K, Nakao K (2000) Kidney produces a novel acylated peptide, ghrelin. FEBS Lett 486(3):213–216
Tena-Sempere M, Barreiro ML, Gonzalez LC, Gaytan F, Zhang FP, Caminos JE, Pinilla L, Casanueva FF, Dieguez C, Aguilar E (2002) Novel expression and functional role of ghrelin in rat testis. Endocrinology 143(2):717–725. https://doi.org/10.1210/endo.143.2.8646
Gualillo O, Caminos J, Blanco M, Garcia-Caballero T, Kojima M, Kangawa K, Dieguez C, Casanueva F (2001) Ghrelin, a novel placental-derived hormone. Endocrinology 142(2):788–794. https://doi.org/10.1210/endo.142.2.7987
Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M (2000) Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 141(11):4255–4261. https://doi.org/10.1210/endo.141.11.7757
Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M (2002) The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 123(4):1120–1128
Wajnrajch MP, Ten I, Gertner JM, Leibel RL (2000) Genomic organization of the human GHRELIN gene. Int J Disabil Hum Dev 1(4):231–234
Seim I, Collet C, Herington AC, Chopin LK (2007) Revised genomic structure of the human ghrelin gene and identification of novel exons, alternative splice variants and natural antisense transcripts. BMC Genom 8:298. https://doi.org/10.1186/1471-2164-8-298
Sato T, Nakamura Y, Shiimura Y, Ohgusu H, Kangawa K, Kojima M (2012) Structure, regulation and function of ghrelin. J Biochem 151(2):119–128. https://doi.org/10.1093/jb/mvr134
Liu B, Garcia EA, Korbonits M (2011) Genetic studies on the ghrelin, growth hormone secretagogue receptor (GHSR) and ghrelin O-acyl transferase (GOAT) genes. Peptides 32(11):2191–2207. https://doi.org/10.1016/j.peptides.2011.09.006
Zhang JV, Ren PG, Avsian-Kretchmer O, Luo CW, Rauch R, Klein C, Hsueh AJ (2005) Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science 310(5750):996–999. https://doi.org/10.1126/science.1117255
Depoortere I (2012) GI functions of GPR39: novel biology. Curr Opin Pharmacol 12(6):647–652. https://doi.org/10.1016/j.coph.2012.07.019
Gargantini E, Grande C, Trovato L, Ghigo E, Granata R (2013) The role of obestatin in glucose and lipid metabolism. Horm Metab Res 45(13):1002–1008. https://doi.org/10.1055/s-0033-1351325
Baragli A, Lanfranco F, Allasia S, Granata R, Ghigo E (2011) Neuroendocrine and metabolic activities of ghrelin gene products. Peptides 32(11):2323–2332. https://doi.org/10.1016/j.peptides.2011.10.024
Gesmundo I, Gallo D, Favaro E, Ghigo E, Granata R (2013) Obestatin: a new metabolic player in the pancreas and white adipose tissue. IUBMB Life 65(12):976–982. https://doi.org/10.1002/iub.1226
Granata R, Gallo D, Luque RM, Baragli A, Scarlatti F, Grande C, Gesmundo I, Cordoba-Chacon J, Bergandi L, Settanni F, Togliatto G, Volante M, Garetto S, Annunziata M, Chanclon B, Gargantini E, Rocchietto S, Matera L, Datta G, Morino M, Brizzi MF, Ong H, Camussi G, Castano JP, Papotti M, Ghigo E (2012) Obestatin regulates adipocyte function and protects against diet-induced insulin resistance and inflammation. FASEB J 26(8):3393–3411. https://doi.org/10.1096/fj.11-201343
Miraglia del Giudice E, Santoro N, Cirillo G, Raimondo P, Grandone A, D’Aniello A, Di Nardo M, Perrone L (2004) Molecular screening of the ghrelin gene in Italian obese children: the Leu72Met variant is associated with an earlier onset of obesity. Int J Obes Relat Metab Disord 28(3):447–450. https://doi.org/10.1038/sj.ijo.0802572
Poykko S, Ukkola O, Kauma H, Savolainen MJ, Kesaniemi YA (2003) Ghrelin Arg51Gln mutation is a risk factor for Type 2 diabetes and hypertension in a random sample of middle-aged subjects. Diabetologia 46(4):455–458. https://doi.org/10.1007/s00125-003-1058-z
Ukkola O, Ravussin E, Jacobson P, Snyder EE, Chagnon M, Sjostrom L, Bouchard C (2001) Mutations in the preproghrelin/ghrelin gene associated with obesity in humans. J Clin Endocrinol Metab 86(8):3996–3999. https://doi.org/10.1210/jcem.86.8.7914
Ukkola O, Ravussin E, Jacobson P, Perusse L, Rankinen T, Tschop M, Heiman ML, Leon AS, Rao DC, Skinner JS, Wilmore JH, Sjostrom L, Bouchard C (2002) Role of ghrelin polymorphisms in obesity based on three different studies. Obes Res 10(8):782–791. https://doi.org/10.1038/oby.2002.106
Hosoda H, Kojima M, Matsuo H, Kangawa K (2000) Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun 279(3):909–913. https://doi.org/10.1006/bbrc.2000.4039
Sakata I, Yang J, Lee CE, Osborne-Lawrence S, Rovinsky SA, Elmquist JK, Zigman JM (2009) Colocalization of ghrelin O-acyltransferase and ghrelin in gastric mucosal cells. Am J Physiol Endocrinol Metab 297(1):E134–E141. https://doi.org/10.1152/ajpendo.90859.2008
Yang J, Zhao TJ, Goldstein JL, Brown MS (2008) Inhibition of ghrelin O-acyltransferase (GOAT) by octanoylated pentapeptides. Proc Natl Acad Sci USA 105(31):10750–10755. https://doi.org/10.1073/pnas.0805353105
Lim CT, Kola B, Grossman A, Korbonits M (2011) The expression of ghrelin O-acyltransferase (GOAT) in human tissues. Endocr J 58(8):707–710
Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, Witcher DR, Luo S, Onyia JE, Hale JE (2008) Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA 105(17):6320–6325. https://doi.org/10.1073/pnas.0800708105
Hosoda H, Kojima M, Mizushima T, Shimizu S, Kangawa K (2003) Structural divergence of human ghrelin. Identification of multiple ghrelin-derived molecules produced by post-translational processing. J Biol Chem 278(1):64–70. https://doi.org/10.1074/jbc.M205366200
Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL (2008) Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 132(3):387–396. https://doi.org/10.1016/j.cell.2008.01.017
Dehlin E, Liu J, Yun SH, Fox E, Snyder S, Gineste C, Willingham L, Geysen M, Gaylinn BD, Sando JJ (2008) Regulation of ghrelin structure and membrane binding by phosphorylation. Peptides 29(6):904–911. https://doi.org/10.1016/j.peptides.2008.02.001
Mundinger TO, Cummings DE, Taborsky GJ Jr (2006) Direct stimulation of ghrelin secretion by sympathetic nerves. Endocrinology 147(6):2893–2901. https://doi.org/10.1210/en.2005-1182
Engelstoft MS, Park WM, Sakata I, Kristensen LV, Husted AS, Osborne-Lawrence S, Piper PK, Walker AK, Pedersen MH, Nohr MK, Pan J, Sinz CJ, Carrington PE, Akiyama TE, Jones RM, Tang C, Ahmed K, Offermanns S, Egerod KL, Zigman JM, Schwartz TW (2013) Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol Metab 2(4):376–392. https://doi.org/10.1016/j.molmet.2013.08.006
Tong J, Dave N, Mugundu GM, Davis HW, Gaylinn BD, Thorner MO, Tschop MH, D’Alessio D, Desai PB (2013) The pharmacokinetics of acyl, des-acyl, and total ghrelin in healthy human subjects. Eur J Endocrinol 168(6):821–828. https://doi.org/10.1530/EJE-13-0072
Liu J, Prudom CE, Nass R, Pezzoli SS, Oliveri MC, Johnson ML, Veldhuis P, Gordon DA, Howard AD, Witcher DR, Geysen HM, Gaylinn BD, Thorner MO (2008) Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J Clin Endocrinol Metab 93(5):1980–1987. https://doi.org/10.1210/jc.2007-2235
Akamizu T, Shinomiya T, Irako T, Fukunaga M, Nakai Y, Nakai Y, Kangawa K (2005) Separate measurement of plasma levels of acylated and desacyl ghrelin in healthy subjects using a new direct ELISA assay. J Clin Endocrinol Metab 90(1):6–9. https://doi.org/10.1210/jc.2004-1640
Janssen S, Laermans J, Verhulst PJ, Thijs T, Tack J, Depoortere I (2011) Bitter taste receptors and alpha-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc Natl Acad Sci USA 108(5):2094–2099. https://doi.org/10.1073/pnas.1011508108
Mizutani M, Atsuchi K, Asakawa A, Matsuda N, Fujimura M, Inui A, Kato I, Fujimiya M (2009) Localization of acyl ghrelin- and des-acyl ghrelin-immunoreactive cells in the rat stomach and their responses to intragastric pH. Am J Physiol Gastrointest Liver Physiol 297(5):G974–G980
De Vriese C, Gregoire F, Lema-Kisoka R, Waelbroeck M, Robberecht P, Delporte C (2004) Ghrelin degradation by serum and tissue homogenates: identification of the cleavage sites. Endocrinology 145(11):4997–5005. https://doi.org/10.1210/en.2004-0569
Banks WA, Tschop M, Robinson SM, Heiman ML (2002) Extent and direction of ghrelin transport across the blood–brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther 302(2):822–827. https://doi.org/10.1124/jpet.102.034827
Banks WA, Burney BO, Robinson SM (2008) Effects of triglycerides, obesity, and starvation on ghrelin transport across the blood–brain barrier. Peptides 29(11):2061–2065. https://doi.org/10.1016/j.peptides.2008.07.001
Barazzoni R, Zanetti M, Stebel M, Biolo G, Cattin L, Guarnieri G (2003) Hyperleptinemia prevents increased plasma ghrelin concentration during short-term moderate caloric restriction in rats. Gastroenterology 124(5):1188–1192
Gualillo O, Caminos JE, Nogueiras R, Seoane LM, Arvat E, Ghigo E, Casanueva FF, Dieguez C (2002) Effect of food restriction on ghrelin in normal-cycling female rats and in pregnancy. Obes Res 10(7):682–687. https://doi.org/10.1038/oby.2002.92
Cummings DE, Overduin J (2007) Gastrointestinal regulation of food intake. J Clin Invest 117(1):13–23. https://doi.org/10.1172/JCI30227
Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS (2001) A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50(8):1714–1719
Tschop M, Wawarta R, Riepl RL, Friedrich S, Bidlingmaier M, Landgraf R, Folwaczny C (2001) Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest 24(6):RC19–R21. https://doi.org/10.1007/BF03351037
Cummings DE, Foster-Schubert KE, Overduin J (2005) Ghrelin and energy balance: focus on current controversies. Curr Drug Targets 6(2):153–169
Sanchez J, Oliver P, Palou A, Pico C (2004) The inhibition of gastric ghrelin production by food intake in rats is dependent on the type of macronutrient. Endocrinology 145(11):5049–5055. https://doi.org/10.1210/en.2004-0493
Otto B, Cuntz U, Fruehauf E, Wawarta R, Folwaczny C, Riepl RL, Heiman ML, Lehnert P, Fichter M, Tschop M (2001) Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur J Endocrinol 145(5):669–673
Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML (2001) Circulating ghrelin levels are decreased in human obesity. Diabetes 50(4):707–709
Barazzoni R, Zanetti M, Ferreira C, Vinci P, Pirulli A, Mucci M, Dore F, Fonda M, Ciocchi B, Cattin L, Guarnieri G (2007) Relationships between desacylated and acylated ghrelin and insulin sensitivity in the metabolic syndrome. J Clin Endocrinol Metab 92(10):3935–3940. https://doi.org/10.1210/jc.2006-2527
Barazzoni R, Zanetti M, Nagliati C, Cattin MR, Ferreira C, Giuricin M, Palmisano S, Edalucci E, Dore F, Guarnieri G, de Manzini N (2013) Gastric bypass does not normalize obesity-related changes in ghrelin profile and leads to higher acylated ghrelin fraction. Obesity (Silver Spring) 21(4):718–722. https://doi.org/10.1002/oby.20272
Kirchner H, Gutierrez JA, Solenberg PJ, Pfluger PT, Czyzyk TA, Willency JA, Schurmann A, Joost HG, Jandacek RJ, Hale JE, Heiman ML, Tschop MH (2009) GOAT links dietary lipids with the endocrine control of energy balance. Nat Med 15(7):741–745. https://doi.org/10.1038/nm.1997
Nishi Y, Hiejima H, Hosoda H, Kaiya H, Mori K, Fukue Y, Yanase T, Nawata H, Kangawa K, Kojima M (2005) Ingested medium-chain fatty acids are directly utilized for the acyl modification of ghrelin. Endocrinology 146(5):2255–2264. https://doi.org/10.1210/en.2004-0695
Barazzoni R, Gortan Cappellari G, Semolic A, Ius M, Dore F, Giacca M, Zanetti M, Vinci P, Guarnieri G (2017) Intravenous lipid infusion and total plasma fatty acids positively modulate plasma acylated ghrelin in vivo. Clin Nutr 36(3):775–781. https://doi.org/10.1016/j.clnu.2016.05.017
Muller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, Batterham RL, Benoit SC, Bowers CY, Broglio F, Casanueva FF, D’Alessio D, Depoortere I, Geliebter A, Ghigo E, Cole PA, Cowley M, Cummings DE, Dagher A, Diano S, Dickson SL, Dieguez C, Granata R, Grill HJ, Grove K, Habegger KM, Heppner K, Heiman ML, Holsen L, Holst B, Inui A, Jansson JO, Kirchner H, Korbonits M, Laferrere B, LeRoux CW, Lopez M, Morin S, Nakazato M, Nass R, Perez-Tilve D, Pfluger PT, Schwartz TW, Seeley RJ, Sleeman M, Sun Y, Sussel L, Tong J, Thorner MO, van der Lely AJ, van der Ploeg LH, Zigman JM, Kojima M, Kangawa K, Smith RG, Horvath T, Tschop MH (2015) Ghrelin. Mol Metab 4(6):437–460. https://doi.org/10.1016/j.molmet.2015.03.005
Bednarek MA, Feighner SD, Pong SS, McKee KK, Hreniuk DL, Silva MV, Warren VA, Howard AD, Van Der Ploeg LH, Heck JV (2000) Structure–function studies on the new growth hormone-releasing peptide, ghrelin: minimal sequence of ghrelin necessary for activation of growth hormone secretagogue receptor 1a. J Med Chem 43(23):4370–4376
Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, Bhattacharya S, Carpenter R, Grossman AB, Korbonits M (2002) The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab 87(6):2988. https://doi.org/10.1210/jcem.87.6.8739
Hattori N, Saito T, Yagyu T, Jiang BH, Kitagawa K, Inagaki C (2001) GH, GH receptor, GH secretagogue receptor, and ghrelin expression in human T cells, B cells, and neutrophils. J Clin Endocrinol Metab 86(9):4284–4291. https://doi.org/10.1210/jcem.86.9.7866
Shuto Y, Shibasaki T, Wada K, Parhar I, Kamegai J, Sugihara H, Oikawa S, Wakabayashi I (2001) Generation of polyclonal antiserum against the growth hormone secretagogue receptor (GHS-R): evidence that the GHS-R exists in the hypothalamus, pituitary and stomach of rats. Life Sci 68(9):991–996
Papotti M, Ghe C, Cassoni P, Catapano F, Deghenghi R, Ghigo E, Muccioli G (2000) Growth hormone secretagogue binding sites in peripheral human tissues. J Clin Endocrinol Metab 85(10):3803–3807. https://doi.org/10.1210/jcem.85.10.6846
Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD (1997) Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res 48(1):23–29
Gershon E, Vale WW (2014) CRF type 2 receptors mediate the metabolic effects of ghrelin in C2C12 cells. Obesity (Silver Spring) 22(2):380–389. https://doi.org/10.1002/oby.20535
McGirr R, McFarland MS, McTavish J, Luyt LG, Dhanvantari S (2011) Design and characterization of a fluorescent ghrelin analog for imaging the growth hormone secretagogue receptor 1a. Regul Pept 172(1–3):69–76. https://doi.org/10.1016/j.regpep.2011.08.011
Moreno M, Chaves JF, Sancho-Bru P, Ramalho F, Ramalho LN, Mansego ML, Ivorra C, Dominguez M, Conde L, Millan C, Mari M, Colmenero J, Lozano JJ, Jares P, Vidal J, Forns X, Arroyo V, Caballeria J, Gines P, Bataller R (2010) Ghrelin attenuates hepatocellular injury and liver fibrogenesis in rodents and influences fibrosis progression in humans. Hepatology 51(3):974–985. https://doi.org/10.1002/hep.23421
Ueberberg B, Unger N, Saeger W, Mann K, Petersenn S (2009) Expression of ghrelin and its receptor in human tissues. Horm Metab Res 41(11):814–821. https://doi.org/10.1055/s-0029-1233462
Filigheddu N, Gnocchi VF, Coscia M, Cappelli M, Porporato PE, Taulli R, Traini S, Baldanzi G, Chianale F, Cutrupi S, Arnoletti E, Ghe C, Fubini A, Surico N, Sinigaglia F, Ponzetto C, Muccioli G, Crepaldi T, Graziani A (2007) Ghrelin and des-acyl ghrelin promote differentiation and fusion of C2C12 skeletal muscle cells. Mol Biol Cell 18(3):986–994. https://doi.org/10.1091/mbc.E06-05-0402
Sun Y, Garcia JM, Smith RG (2007) Ghrelin and growth hormone secretagogue receptor expression in mice during aging. Endocrinology 148(3):1323–1329. https://doi.org/10.1210/en.2006-0782
Callaghan B, Furness JB (2014) Novel and conventional receptors for ghrelin, desacyl-ghrelin, and pharmacologically related compounds. Pharmacol Rev 66(4):984–1001. https://doi.org/10.1124/pr.113.008433
Muccioli G, Baragli A, Granata R, Papotti M, Ghigo E (2007) Heterogeneity of ghrelin/growth hormone secretagogue receptors. Toward the understanding of the molecular identity of novel ghrelin/GHS receptors. Neuroendocrinology 86(3):147–164. https://doi.org/10.1159/000105141
Dardzinska JA, Malgorzewicz S, Kaska L, Proczko M, Stefaniak T, Stankiewicz M, Sledzinski Z (2014) Fasting and postprandial acyl and desacyl ghrelin levels in obese and non-obese subjects. Endokrynol Pol 65(5):377–381. https://doi.org/10.5603/EP.2014.0052
Gil-Campos M, Aguilera CM, Canete R, Gil A (2006) Ghrelin: a hormone regulating food intake and energy homeostasis. Br J Nutr 96(2):201–226
Barazzoni R, Gortan Cappellari G, Zanetti M, Guarnieri G (2012) Ghrelin and muscle metabolism in chronic uremia. J Ren Nutr 22(1):171–175. https://doi.org/10.1053/j.jrn.2011.10.017
Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S (2001) A role for ghrelin in the central regulation of feeding. Nature 409(6817):194–198. https://doi.org/10.1038/35051587
Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, Batterham RL, Taheri S, Stanley SA, Ghatei MA, Bloom SR (2001) Ghrelin causes hyperphagia and obesity in rats. Diabetes 50(11):2540–2547
Tschop M, Smiley DL, Heiman ML (2000) Ghrelin induces adiposity in rodents. Nature 407(6806):908–913. https://doi.org/10.1038/35038090
Dornonville de la Cour C, Lindqvist A, Egecioglu E, Tung YC, Surve V, Ohlsson C, Jansson JO, Erlanson-Albertsson C, Dickson SL, Hakanson R (2005) Ghrelin treatment reverses the reduction in weight gain and body fat in gastrectomised mice. Gut 54(7):907–913. https://doi.org/10.1136/gut.2004.058578
Adachi S, Takiguchi S, Okada K, Yamamoto K, Yamasaki M, Miyata H, Nakajima K, Fujiwara Y, Hosoda H, Kangawa K, Mori M, Doki Y (2010) Effects of ghrelin administration after total gastrectomy: a prospective, randomized, placebo-controlled phase II study. Gastroenterology 138(4):1312–1320. https://doi.org/10.1053/j.gastro.2009.12.058
Arnold M, Mura A, Langhans W, Geary N (2006) Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci 26(43):11052–11060. https://doi.org/10.1523/JNEUROSCI.2606-06.2006
Kola B, Farkas I, Christ-Crain M, Wittmann G, Lolli F, Amin F, Harvey-White J, Liposits Z, Kunos G, Grossman AB, Fekete C, Korbonits M (2008) The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PloS One 3(3):e1797. https://doi.org/10.1371/journal.pone.0001797
Bagnasco M, Dube MG, Kalra PS, Kalra SP (2002) Evidence for the existence of distinct central appetite, energy expenditure, and ghrelin stimulation pathways as revealed by hypothalamic site-specific leptin gene therapy. Endocrinology 143(11):4409–4421
Wang L, Saint-Pierre DH, Tache Y (2002) Peripheral ghrelin selectively increases Fos expression in neuropeptide Y-synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci Lett 325(1):47–51
Willesen MG, Kristensen P, Romer J (1999) Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology 70(5):306–316
Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK (2006) Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol 494(3):528–548. https://doi.org/10.1002/cne.20823
Malik S, McGlone F, Bedrossian D, Dagher A (2008) Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab 7(5):400–409. https://doi.org/10.1016/j.cmet.2008.03.007
Asakawa A, Inui A, Fujimiya M, Sakamaki R, Shinfuku N, Ueta Y, Meguid MM, Kasuga M (2005) Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut 54(1):18–24. https://doi.org/10.1136/gut.2004.038737
Inhoff T, Monnikes H, Noetzel S, Stengel A, Goebel M, Dinh QT, Riedl A, Bannert N, Wisser AS, Wiedenmann B, Klapp BF, Tache Y, Kobelt P (2008) Desacyl ghrelin inhibits the orexigenic effect of peripherally injected ghrelin in rats. Peptides 29(12):2159–2168. https://doi.org/10.1016/j.peptides.2008.09.014
Toshinai K, Yamaguchi H, Sun Y, Smith RG, Yamanaka A, Sakurai T, Date Y, Mondal MS, Shimbara T, Kawagoe T, Murakami N, Miyazato M, Kangawa K, Nakazato M (2006) Des-acyl ghrelin induces food intake by a mechanism independent of the growth hormone secretagogue receptor. Endocrinology 147(5):2306–2314. https://doi.org/10.1210/en.2005-1357
Barazzoni R, Deutz NEP, Biolo G, Bischoff S, Boirie Y, Cederholm T, Cuerda C, Delzenne N, Leon Sanz M, Ljungqvist O, Muscaritoli M, Pichard C, Preiser JC, Sbraccia P, Singer P, Tappy L, Thorens B, Van Gossum A, Vettor R, Calder PC (2017) Carbohydrates and insulin resistance in clinical nutrition: recommendations from the ESPEN expert group. Clin Nutr 36(2):355–363. https://doi.org/10.1016/j.clnu.2016.09.010
Broglio F, Arvat E, Benso A, Gottero C, Muccioli G, Papotti M, van der Lely AJ, Deghenghi R, Ghigo E (2001) Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab 86(10):5083–5086. https://doi.org/10.1210/jcem.86.10.8098
Tong J, Prigeon RL, Davis HW, Bidlingmaier M, Kahn SE, Cummings DE, Tschop MH, D’Alessio D (2010) Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes 59(9):2145–2151. https://doi.org/10.2337/db10-0504
Sun Y, Butte NF, Garcia JM, Smith RG (2008) Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology 149(2):843–850. https://doi.org/10.1210/en.2007-0271
Dezaki K, Kakei M, Yada T (2007) Ghrelin uses Galphai2 and activates voltage-dependent K+ channels to attenuate glucose-induced Ca2+ signaling and insulin release in islet beta-cells: novel signal transduction of ghrelin. Diabetes 56(9):2319–2327. https://doi.org/10.2337/db07-0345
Poykko SM, Kellokoski E, Horkko S, Kauma H, Kesaniemi YA, Ukkola O (2003) Low plasma ghrelin is associated with insulin resistance, hypertension, and the prevalence of type 2 diabetes. Diabetes 52(10):2546–2553
Barazzoni R, Zanetti M, Stulle M, Mucci MP, Pirulli A, Dore F, Panzetta G, Vasile A, Biolo G, Guarnieri G (2008) Higher total ghrelin levels are associated with higher insulin-mediated glucose disposal in non-diabetic maintenance hemodialysis patients. Clin Nutr 27(1):142–149. https://doi.org/10.1016/j.clnu.2007.06.013
McLaughlin T, Abbasi F, Lamendola C, Frayo RS, Cummings DE (2004) Plasma ghrelin concentrations are decreased in insulin-resistant obese adults relative to equally obese insulin-sensitive controls. J Clin Endocrinol Metab 89(4):1630–1635. https://doi.org/10.1210/jc.2003-031572
Zanetti M, Gortan Cappellari G, Semolic A, Burekovic I, Fonda M, Cattin L, Barazzoni R (2017) Gender-specific association of desacylated ghrelin with subclinical atherosclerosis in the metabolic syndrome. Arch Med Res. https://doi.org/10.1016/j.arcmed.2017.09.002
Broglio F, Gottero C, Prodam F, Gauna C, Muccioli G, Papotti M, Abribat T, Van Der Lely AJ, Ghigo E (2004) Non-acylated ghrelin counteracts the metabolic but not the neuroendocrine response to acylated ghrelin in humans. J Clin Endocrinol Metab 89(6):3062–3065. https://doi.org/10.1210/jc.2003-031964
Gauna C, Kiewiet RM, Janssen JA, van de Zande B, Delhanty PJ, Ghigo E, Hofland LJ, Themmen AP, van der Lely AJ (2007) Unacylated ghrelin acts as a potent insulin secretagogue in glucose-stimulated conditions. Am J Physiol Endocrinol Metab 293(3):E697–E704. https://doi.org/10.1152/ajpendo.00219.2007
Barazzoni R, Gortan Cappellari G, Semolic A, Chendi E, Ius M, Situlin R, Zanetti M, Vinci P, Guarnieri G (2014) The association between hematological parameters and insulin resistance is modified by body mass index—results from the North-East Italy MoMa population study. PloS One 9(7):e101590. https://doi.org/10.1371/journal.pone.0101590
Barazzoni R, Gortan Cappellari G, Semolic A, Ius M, Mamolo L, Dore F, Giacca M, Zanetti M, Vinci P, Guarnieri G (2016) Plasma total and unacylated ghrelin predict 5-year changes in insulin resistance. Clin Nutr 35(5):1168–1173. https://doi.org/10.1016/j.clnu.2015.10.002
Granata R, Settanni F, Biancone L, Trovato L, Nano R, Bertuzzi F, Destefanis S, Annunziata M, Martinetti M, Catapano F, Ghe C, Isgaard J, Papotti M, Ghigo E, Muccioli G (2007) Acylated and unacylated ghrelin promote proliferation and inhibit apoptosis of pancreatic beta-cells and human islets: involvement of 3′,5′-cyclic adenosine monophosphate/protein kinase A, extracellular signal-regulated kinase 1/2, and phosphatidyl inositol 3-kinase/Akt signaling. Endocrinology 148(2):512–529. https://doi.org/10.1210/en.2006-0266
Gauna C, Delhanty PJ, van Aken MO, Janssen JA, Themmen AP, Hofland LJ, Culler M, Broglio F, Ghigo E, van der Lely AJ (2006) Unacylated ghrelin is active on the INS-1E rat insulinoma cell line independently of the growth hormone secretagogue receptor type 1a and the corticotropin releasing factor 2 receptor. Mol Cell Endocrinol 251(1–2):103–111. https://doi.org/10.1016/j.mce.2006.03.040
Tong J, Davis HW, Summer S, Benoit SC, Haque A, Bidlingmaier M, Tschop MH, D’Alessio D (2014) Acute administration of unacylated ghrelin has no effect on Basal or stimulated insulin secretion in healthy humans. Diabetes 63(7):2309–2319. https://doi.org/10.2337/db13-1598
Heijboer AC, van den Hoek AM, Parlevliet ET, Havekes LM, Romijn JA, Pijl H, Corssmit EP (2006) Ghrelin differentially affects hepatic and peripheral insulin sensitivity in mice. Diabetologia 49(4):732–738. https://doi.org/10.1007/s00125-006-0138-2
Barazzoni R, Bosutti A, Stebel M, Cattin MR, Roder E, Visintin L, Cattin L, Biolo G, Zanetti M, Guarnieri G (2005) Ghrelin regulates mitochondrial-lipid metabolism gene expression and tissue fat distribution in liver and skeletal muscle. Am J Physiol Endocrinol Metab 288(1):E228–E235. https://doi.org/10.1152/ajpendo.00115.2004
Pardo M, Roca-Rivada A, Al-Massadi O, Seoane LM, Camina JP, Casanueva FF (2010) Peripheral leptin and ghrelin receptors are regulated in a tissue-specific manner in activity-based anorexia. Peptides 31(10):1912–1919. https://doi.org/10.1016/j.peptides.2010.06.022
van Thuijl H, Kola B, Korbonits M (2008) Appetite and metabolic effects of ghrelin and cannabinoids: involvement of AMP-activated protein kinase. Vitam Horm 77:121–148. https://doi.org/10.1016/S0083-6729(06)77006-6
Gauna C, Delhanty PJ, Hofland LJ, Janssen JA, Broglio F, Ross RJ, Ghigo E, van der Lely AJ (2005) Ghrelin stimulates, whereas des-octanoyl ghrelin inhibits, glucose output by primary hepatocytes. J Clin Endocrinol Metab 90(2):1055–1060. https://doi.org/10.1210/jc.2004-1069
Barazzoni R, Zanetti M, Cattin MR, Visintin L, Vinci P, Cattin L, Stebel M, Guarnieri G (2007) Ghrelin enhances in vivo skeletal muscle but not liver AKT signaling in rats. Obesity 15(11):2614–2623. https://doi.org/10.1038/oby.2007.313
Obay BD, Tasdemir E, Tumer C, Bilgin H, Atmaca M (2008) Dose dependent effects of ghrelin on pentylenetetrazole-induced oxidative stress in a rat seizure model. Peptides 29(3):448–455. https://doi.org/10.1016/j.peptides.2007.11.020
Li Y, Hai J, Li L, Chen X, Peng H, Cao M, Zhang Q (2013) Administration of ghrelin improves inflammation, oxidative stress, and apoptosis during and after non-alcoholic fatty liver disease development. Endocrine 43(2):376–386. https://doi.org/10.1007/s12020-012-9761-5
Barazzoni R, Semolic A, Cattin MR, Zanetti M, Guarnieri G (2014) Acylated ghrelin limits fat accumulation and improves redox state and inflammation markers in the liver of high-fat-fed rats. Obesity (Silver Spring) 22(1):170–177. https://doi.org/10.1002/oby.20454
Murata M, Okimura Y, Iida K, Matsumoto M, Sowa H, Kaji H, Kojima M, Kangawa K, Chihara K (2002) Ghrelin modulates the downstream molecules of insulin signaling in hepatoma cells. J Biol Chem 277(7):5667–5674. https://doi.org/10.1074/jbc.M103898200
Liu HY, Hong T, Wen GB, Han J, Zuo D, Liu Z, Cao W (2009) Increased basal level of Akt-dependent insulin signaling may be responsible for the development of insulin resistance. Am J Physiol Endocrinol Metab 297(4):E898–E906. https://doi.org/10.1152/ajpendo.00374.2009
Gortan Cappellari G, Zanetti M, Semolic A, Vinci P, Ruozi G, Falcione A, Filigheddu N, Guarnieri G, Graziani A, Giacca M, Barazzoni R (2016) Unacylated ghrelin reduces skeletal muscle reactive oxygen species generation and inflammation and prevents high-fat diet-induced hyperglycemia and whole-body insulin resistance in rodents. Diabetes 65(4):874–886. https://doi.org/10.2337/db15-1019
Gortan Cappellari G, Zanetti M, Semolic A, Vinci P, Ruozi G, De Nardo M, Filigheddu N, Guarnieri G, Giacca M, Graziani A (2015) Unacylated ghrelin does not alter mitochondrial function, redox state and triglyceride content in rat liver in vivo. Clin Nutr Exp 4:1–7
Rossetti A, Togliatto G, Rolo AP, Teodoro JS, Granata R, Ghigo E, Columbano A, Palmeira CM, Brizzi MF (2017) Unacylated ghrelin prevents mitochondrial dysfunction in a model of ischemia/reperfusion liver injury. Cell Death Discov 3:17077. https://doi.org/10.1038/cddiscovery.2017.77
Davies JS, Kotokorpi P, Eccles SR, Barnes SK, Tokarczuk PF, Allen SK, Whitworth HS, Guschina IA, Evans BA, Mode A, Zigman JM, Wells T (2009) Ghrelin induces abdominal obesity via GHS-R-dependent lipid retention. Mol Endocrinol 23(6):914–924. https://doi.org/10.1210/me.2008-0432
Muccioli G, Pons N, Ghe C, Catapano F, Granata R, Ghigo E (2004) Ghrelin and des-acyl ghrelin both inhibit isoproterenol-induced lipolysis in rat adipocytes via a non-type 1a growth hormone secretagogue receptor. Eur J Pharmacol 498(1–3):27–35. https://doi.org/10.1016/j.ejphar.2004.07.066
Perez-Tilve D, Heppner K, Kirchner H, Lockie SH, Woods SC, Smiley DL, Tschop M, Pfluger P (2011) Ghrelin-induced adiposity is independent of orexigenic effects. FASEB J 25(8):2814–2822. https://doi.org/10.1096/fj.11-183632
Wortley KE, del Rincon JP, Murray JD, Garcia K, Iida K, Thorner MO, Sleeman MW (2005) Absence of ghrelin protects against early-onset obesity. J Clin Invest 115(12):3573–3578. https://doi.org/10.1172/JCI26003
Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD, Williams TD, Lachey JL, Seeley RJ, Lowell BB, Elmquist JK (2005) Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest 115(12):3564–3572. https://doi.org/10.1172/JCI26002
Kim MS, Yoon CY, Jang PG, Park YJ, Shin CS, Park HS, Ryu JW, Pak YK, Park JY, Lee KU, Kim SY, Lee HK, Kim YB, Park KS (2004) The mitogenic and antiapoptotic actions of ghrelin in 3T3-L1 adipocytes. Mol Endocrinol 18(9):2291–2301. https://doi.org/10.1210/me.2003-0459
Choi K, Roh SG, Hong YH, Shrestha YB, Hishikawa D, Chen C, Kojima M, Kangawa K, Sasaki S (2003) The role of ghrelin and growth hormone secretagogues receptor on rat adipogenesis. Endocrinology 144(3):754–759. https://doi.org/10.1210/en.2002-220783
Andrews ZB, Erion DM, Beiler R, Choi CS, Shulman GI, Horvath TL (2010) Uncoupling protein-2 decreases the lipogenic actions of ghrelin. Endocrinology 151(5):2078–2086. https://doi.org/10.1210/en.2009-0850
Miegueu P, St Pierre D, Broglio F, Cianflone K (2011) Effect of desacyl ghrelin, obestatin and related peptides on triglyceride storage, metabolism and GHSR signaling in 3T3-L1 adipocytes. J Cell Biochem 112(2):704–714. https://doi.org/10.1002/jcb.22983
Zhang W, Chai B, Li JY, Wang H, Mulholland MW (2008) Effect of des-acyl ghrelin on adiposity and glucose metabolism. Endocrinology 149(9):4710–4716. https://doi.org/10.1210/en.2008-0263
Barazzoni R, Zanetti M, Gortan Cappellari G, Semolic A, Boschelle M, Codarin E, Pirulli A, Cattin L, Guarnieri G (2012) Fatty acids acutely enhance insulin-induced oxidative stress and cause insulin resistance by increasing mitochondrial reactive oxygen species (ROS) generation and nuclear factor-kappaB inhibitor (IkappaB)-nuclear factor-kappaB (NFkappaB) activation in rat muscle, in the absence of mitochondrial dysfunction. Diabetologia 55(3):773–782. https://doi.org/10.1007/s00125-011-2396-x
Zanetti M, Barazzoni R, Guarnieri G (2008) Inflammation and insulin resistance in uremia. J Ren Nutr 18(1):70–75. https://doi.org/10.1053/j.jrn.2007.10.015
Barazzoni R (2004) Skeletal muscle mitochondrial protein metabolism and function in ageing and type 2 diabetes. Curr Opin Clin Nutr Metab Care 7(1):97–102
Guarnieri G, Zanetti M, Vinci P, Cattin MR, Barazzoni R (2009) Insulin resistance in chronic uremia. J Ren Nutr 19(1):20–24. https://doi.org/10.1053/j.jrn.2008.11.014
Gortan Cappellari G, Zanetti M, Vinci P, Guarnieri G, Barazzoni R (2017) Unacylated ghrelin: a novel regulator of muscle intermediate metabolism with potential beneficial effects in chronic kidney disease. J Ren Nutr 27(6):474–477. https://doi.org/10.1053/j.jrn.2017.05.005
Wei Y, Sowers JR, Clark SE, Li W, Ferrario CM, Stump CS (2008) Angiotensin II-induced skeletal muscle insulin resistance mediated by NF-kappaB activation via NADPH oxidase. Am J Physiol Endocrinol Metab 294(2):E345–E351. https://doi.org/10.1152/ajpendo.00456.2007
Cai D, Frantz JD, Tawa NE Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE (2004) IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 119(2):285–298. https://doi.org/10.1016/j.cell.2004.09.027
Schenk S, Saberi M, Olefsky JM (2008) Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest 118(9):2992–3002. https://doi.org/10.1172/JCI34260
Morino K, Petersen KF, Shulman GI (2006) Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 55 Suppl 2:S9–S15. https://doi.org/10.2337/diabetes
Saleh MC, Wheeler MB, Chan CB (2002) Uncoupling protein-2: evidence for its function as a metabolic regulator. Diabetologia 45(2):174–187. https://doi.org/10.1007/s00125-001-0737-x
Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD (2002) Superoxide activates mitochondrial uncoupling proteins. Nature 415(6867):96–99. https://doi.org/10.1038/415096a
Barazzoni R, Zanetti M, Bosutti A, Biolo G, Vitali-Serdoz L, Stebel M, Guarnieri G (2005) Moderate caloric restriction, but not physiological hyperleptinemia per se, enhances mitochondrial oxidative capacity in rat liver and skeletal muscle–tissue-specific impact on tissue triglyceride content and AKT activation. Endocrinology 146(4):2098–2106. https://doi.org/10.1210/en.2004-1396
Barazzoni R, Zhu X, Deboer M, Datta R, Culler MD, Zanetti M, Guarnieri G, Marks DL (2010) Combined effects of ghrelin and higher food intake enhance skeletal muscle mitochondrial oxidative capacity and AKT phosphorylation in rats with chronic kidney disease. Kidney Int 77(1):23–28. https://doi.org/10.1038/ki.2009.411
Barazzoni R, Gortan Cappellari G, Palus S, Vinci P, Ruozi G, Zanetti M, Semolic A, Ebner N, von Heahling S, Sinagra G, Giacca M, Springer J (2017) Acylated ghrelin treatment normalizes skeletal muscle mitochondrial oxidative capacity and AKT phosphorylation in rat chronic heart failure. J Cachex Sarcopenia Muscle. https://doi.org/10.1002/jcsm.12254
Suematsu M, Katsuki A, Sumida Y, Gabazza EC, Murashima S, Matsumoto K, Kitagawa N, Akatsuka H, Hori Y, Nakatani K, Togashi K, Yano Y, Adachi Y (2005) Decreased circulating levels of active ghrelin are associated with increased oxidative stress in obese subjects. Eur J Endocrinol 153(3):403–407. https://doi.org/10.1530/eje.1.01977
Omrani H, Alipour MR, Mohaddes G (2015) Ghrelin improves antioxidant defense in blood and brain in normobaric hypoxia in adult male rats. Adv Pharm Bull 5(2):283–288. https://doi.org/10.15171/apb.2015.039
El Eter E, Al Tuwaijiri A, Hagar H, Arafa M (2007) In vivo and in vitro antioxidant activity of ghrelin: attenuation of gastric ischemic injury in the rat. J Gastroenterol Hepatol 22(11):1791–1799. https://doi.org/10.1111/j.1440-1746.2006.04696.x
Iseri SO, Sener G, Yuksel M, Contuk G, Cetinel S, Gedik N, Yegen BC (2005) Ghrelin against alendronate-induced gastric damage in rats. J Endocrinol 187(3):399–406. https://doi.org/10.1677/joe.1.06432
Chang L, Ren Y, Liu X, Li WG, Yang J, Geng B, Weintraub NL, Tang C (2004) Protective effects of ghrelin on ischemia/reperfusion injury in the isolated rat heart. J Cardiovasc Pharmacol 43(2):165–170
Kheradmand A, Alirezaei M, Birjandi M (2010) Ghrelin promotes antioxidant enzyme activity and reduces lipid peroxidation in the rat ovary. Regul Pept 162(1–3):84–89. https://doi.org/10.1016/j.regpep.2010.02.008
Zwirska-Korczala K, Adamczyk-Sowa M, Sowa P, Pilc K, Suchanek R, Pierzchala K, Namyslowski G, Misiolek M, Sodowski K, Kato I, Kuwahara A, Zabielski R (2007) Role of leptin, ghrelin, angiotensin II and orexins in 3T3 L1 preadipocyte cells proliferation and oxidative metabolism. J Physiol Pharmacol 58(Suppl 1):53–64
Neamati S, Alirezaei M, Kheradmand A (2011) Ghrelin acts as an antioxidant agent in the rat kidney. Int J Pept Res Ther 17(3):239–245
Barazzoni R, Zanetti M, Semolic A, Cattin MR, Pirulli A, Cattin L, Guarnieri G (2011) High-fat diet with acyl-ghrelin treatment leads to weight gain with low inflammation, high oxidative capacity and normal triglycerides in rat muscle. PLoS One 6(10):e26224. https://doi.org/10.1371/journal.pone.0026224
Ruozi G, Bortolotti F, Falcione A, Dal Ferro M, Ukovich L, Macedo A, Zentilin L, Filigheddu N, Gortan Cappellari G, Baldini G, Zweyer M, Barazzoni R, Graziani A, Zacchigna S, Giacca M (2015) AAV-mediated in vivo functional selection of tissue-protective factors against ischaemia. Nat Commun 6:7388. https://doi.org/10.1038/ncomms8388
Togliatto G, Trombetta A, Dentelli P, Cotogni P, Rosso A, Tschop MH, Granata R, Ghigo E, Brizzi MF (2013) Unacylated ghrelin promotes skeletal muscle regeneration following hindlimb ischemia via SOD-2-mediated miR-221/222 expression. J Am Heart Assoc 2(6):e000376. https://doi.org/10.1161/JAHA.113.000376
Togliatto G, Trombetta A, Dentelli P, Gallo S, Rosso A, Cotogni P, Granata R, Falcioni R, Delale T, Ghigo E, Brizzi MF (2015) Unacylated ghrelin induces oxidative stress resistance in a glucose intolerance and peripheral artery disease mouse model by restoring endothelial cell miR-126 expression. Diabetes 64(4):1370–1382. https://doi.org/10.2337/db14-0991
Gortan Cappellari G, Semolic A, Ruozi G, Vinci P, Guarnieri G, Bortolotti F, Barbetta D, Zanetti M, Giacca M, Barazzoni R (2017) Unacylated ghrelin normalizes skeletal muscle oxidative stress and prevents muscle catabolism by enhancing tissue mitophagy in experimental chronic kidney disease. FASEB J. https://doi.org/10.1096/fj.201700126R
Baatar D, Patel K, Taub DD (2011) The effects of ghrelin on inflammation and the immune system. Mol Cell Endocrinol 340(1):44–58. https://doi.org/10.1016/j.mce.2011.04.019
Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, Lillard JW Jr, Taub DD (2004) Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Investig 114(1):57–66. https://doi.org/10.1172/JCI21134
Yilmaz Z, Ilcol YO, Ulus IH (2008) Endotoxin increases plasma leptin and ghrelin levels in dogs. Crit Care Med 36(3):828–833. https://doi.org/10.1097/01.CCM.0B013E3181611F5AA
Vila G, Maier C, Riedl M, Nowotny P, Ludvik B, Luger A, Clodi M (2007) Bacterial endotoxin induces biphasic changes in plasma ghrelin in healthy humans. J Clin Endocrinol Metab 92(10):3930–3934. https://doi.org/10.1210/jc.2007-1194
Waseem T, Duxbury M, Ito H, Ashley SW, Robinson MK (2008) Exogenous ghrelin modulates release of pro-inflammatory and anti-inflammatory cytokines in LPS-stimulated macrophages through distinct signaling pathways. Surgery 143(3):334–342. https://doi.org/10.1016/j.surg.2007.09.039
Gonzalez-Rey E, Chorny A, Delgado M (2006) Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology 130(6):1707–1720. https://doi.org/10.1053/j.gastro.2006.01.041
Kodama T, Ashitani J, Matsumoto N, Kangawa K, Nakazato M (2008) Ghrelin treatment suppresses neutrophil-dominant inflammation in airways of patients with chronic respiratory infection. Pulm Pharmacol Ther 21(5):774–779. https://doi.org/10.1016/j.pupt.2008.05.001
Takata A, Takiguchi S, Miyazaki Y, Miyata H, Takahashi T, Kurokawa Y, Yamasaki M, Nakajima K, Mori M, Kangawa K, Doki Y (2015) Randomized phase II study of the anti-inflammatory effect of ghrelin during the postoperative period of esophagectomy. Ann Surg 262(2):230–236. https://doi.org/10.1097/SLA.0000000000000986
Valerio A, Cardile A, Cozzi V, Bracale R, Tedesco L, Pisconti A, Palomba L, Cantoni O, Clementi E, Moncada S, Carruba MO, Nisoli E (2006) TNF-alpha downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. J Clin Investig 116(10):2791–2798. https://doi.org/10.1172/JCI28570
Han D, Huang W, Ma S, Chen J, Gao L, Liu T, Zhang R, Li X, Li C, Fan M, Chen Y, Cao F (2015) Ghrelin improves functional survival of engrafted adipose-derived mesenchymal stem cells in ischemic heart through PI3K/Akt signaling pathway. BioMed Res Int 2015:858349. https://doi.org/10.1155/2015/858349
Hao XK, Wu W, Wang CX, Xie GB, Li T, Wu HM, Huang LT, Zhou ML, Hang CH, Shi JX (2014) Ghrelin alleviates early brain injury after subarachnoid hemorrhage via the PI3K/Akt signaling pathway. Brain Res 1587:15–22. https://doi.org/10.1016/j.brainres.2014.08.069
Waseem T, Duxbury M, Ashley SW, Robinson MK (2014) Ghrelin promotes intestinal epithelial cell proliferation through PI3K/Akt pathway and EGFR trans-activation both converging to ERK 1/2 phosphorylation. Peptides 52:113–121. https://doi.org/10.1016/j.peptides.2013.11.021
Yang D, Liu Z, Zhang H, Luo Q (2013) Ghrelin protects human pulmonary artery endothelial cells against hypoxia-induced injury via PI3-kinase/Akt. Peptides 42:112–117. https://doi.org/10.1016/j.peptides.2013.01.012
Chen X, Chen Q, Wang L, Li G (2013) Ghrelin induces cell migration through GHSR1a-mediated PI3K/Akt/eNOS/NO signaling pathway in endothelial progenitor cells. Metab Clin Exp 62(5):743–752. https://doi.org/10.1016/j.metabol.2012.09.014
Vestergaard ET, Djurhuus CB, Gjedsted J, Nielsen S, Moller N, Holst JJ, Jorgensen JO, Schmitz O (2008) Acute effects of ghrelin administration on glucose and lipid metabolism. J Clin Endocrinol Metab 93(2):438–444. https://doi.org/10.1210/jc.2007-2018
Vestergaard ET, Buhl M, Gjedsted J, Madsen M, Jessen N, Nielsen S, Gaylinn BD, Liu J, Thorner MO, Moller N, Jorgensen JO (2011) Acute peripheral metabolic effects of intraarterial ghrelin infusion in healthy young men. J Clin Endocrinol Metab 96(2):468–477. https://doi.org/10.1210/jc.2010-1995
Lear PV, Iglesias MJ, Feijoo-Bandin S, Rodriguez-Penas D, Mosquera-Leal A, Garcia-Rua V, Gualillo O, Ghe C, Arnoletti E, Muccioli G, Dieguez C, Gonzalez-Juanatey JR, Lago F (2010) Des-acyl ghrelin has specific binding sites and different metabolic effects from ghrelin in cardiomyocytes. Endocrinology 151(7):3286–3298. https://doi.org/10.1210/en.2009-1205
Kim I, Rodriguez-Enriquez S, Lemasters JJ (2007) Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys 462(2):245–253. https://doi.org/10.1016/j.abb.2007.03.034
Klionsky DJ, Emr SD (2000) Autophagy as a regulated pathway of cellular degradation. Science 290(5497):1717–1721
Slupecka M, Wolinski J, Pierzynowski SG (2012) The effects of enteral ghrelin administration on the remodeling of the small intestinal mucosa in neonatal piglets. Regul Pept 174(1–3):38–45. https://doi.org/10.1016/j.regpep.2011.11.007
Tong XX, Wu D, Wang X, Chen HL, Chen JX, Wang XX, Wang XL, Gan L, Guo ZY, Shi GX, Zhang YZ, Jiang W (2012) Ghrelin protects against cobalt chloride-induced hypoxic injury in cardiac H9c2 cells by inhibiting oxidative stress and inducing autophagy. Peptides 38(2):217–227. https://doi.org/10.1016/j.peptides.2012.06.020
Tam BT, Pei XM, Yung BY, Yip SP, Chan LW, Wong CS, Siu PM (2015) Unacylated ghrelin restores insulin and autophagic signaling in skeletal muscle of diabetic mice. Pflug Arch 467(12):2555–2569. https://doi.org/10.1007/s00424-015-1721-5
Kuppens RJ, Delhanty PJ, Huisman TM, van der Lely AJ, Hokken-Koelega AC (2016) Acylated and unacylated ghrelin during OGTT in Prader–Willi syndrome: support for normal response to food intake. Clin Endocrinol. https://doi.org/10.1111/cen.13036
Choe YH, Song SY, Paik KH, Oh YJ, Chu SH, Yeo SH, Kwon EK, Kim EM, Rha MY, Jin DK (2005) Increased density of ghrelin-expressing cells in the gastric fundus and body in Prader–Willi syndrome. J Clin Endocrinol Metab 90(9):5441–5445. https://doi.org/10.1210/jc.2004-1935
Cummings DE, Clement K, Purnell JQ, Vaisse C, Foster KE, Frayo RS, Schwartz MW, Basdevant A, Weigle DS (2002) Elevated plasma ghrelin levels in Prader Willi syndrome. Nat Med 8(7):643–644. https://doi.org/10.1038/nm0702-643
Nicholls RD, Knepper JL (2001) Genome organization, function, and imprinting in Prader–Willi and Angelman syndromes. Annu Rev Genom Hum Genet 2:153–175. https://doi.org/10.1146/annurev.genom.2.1.153
Gagnon J, Zhu L, Anini Y, Wang Q (2015) Neutralizing circulating ghrelin by expressing a growth hormone secretagogue receptor-based protein protects against high-fat diet-induced obesity in mice. Gene Ther 22(9):750–757. https://doi.org/10.1038/gt.2015.38
Wiedmer P, Nogueiras R, Broglio F, D’Alessio D, Tschop MH (2007) Ghrelin, obesity and diabetes. Nat Clin Pract Endocrinol Metab 3(10):705–712. https://doi.org/10.1038/ncpendmet0625
Wortley KE, Anderson KD, Garcia K, Murray JD, Malinova L, Liu R, Moncrieffe M, Thabet K, Cox HJ, Yancopoulos GD, Wiegand SJ, Sleeman MW (2004) Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc Natl Acad Sci USA 101(21):8227–8232. https://doi.org/10.1073/pnas.0402763101
McFarlane MR, Brown MS, Goldstein JL, Zhao TJ (2014) Induced ablation of ghrelin cells in adult mice does not decrease food intake, body weight, or response to high-fat diet. Cell Metab 20(1):54–60. https://doi.org/10.1016/j.cmet.2014.04.007
Sbraccia P, Nisoli E, Barazzoni R (2018) Obesity: focus on ongoing multidisciplinary and comprehensive research. Eat Weight Disord 23(1):1. https://doi.org/10.1007/s40519-017-0462-1
Barazzoni R, Gortan Cappellari G, Ragni M, Nisoli E (2018) Insulin resistance in obesity: an overview of fundamental alterations. Eat Weight Disord 23(2):149–157. https://doi.org/10.1007/s40519-018-0481-6
Barazzoni R, Bischoff SC, Boirie Y, Busetto L, Cederholm T, Dicker D, Toplak H, Van Gossum A, Yumuk V, Vettor R (2018) Sarcopenic obesity: time to meet the challenge. Clin Nutr. https://doi.org/10.1016/j.clnu.2018.04.018
Barazzoni R, Gortan Cappellari G, Semolic A, Ius M, Zanetti M, Gabrielli A, Vinci P, Guarnieri G, Simon G (2018) Central adiposity markers, plasma lipid profile and cardiometabolic risk prediction in overweight-obese individuals. Clin Nutr. https://doi.org/10.1016/j.clnu.2018.04.014
Barazzoni R, Gortan Cappellari G, Semolic A, Ius M, Mamolo L, Dore F, Giacca M, Zanetti M, Vinci P, Guarnieri G (2015) Plasma total and unacylated ghrelin predict 5-year changes in insulin resistance. Clin Nutr. https://doi.org/10.1016/j.clnu.2015.10.002
Gual P, Le Marchand-Brustel Y, Tanti JF (2005) Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie 87(1):99–109. https://doi.org/10.1016/j.biochi.2004.10.019
Delhanty PJ, Huisman M, Baldeon-Rojas LY, van den Berge I, Grefhorst A, Abribat T, Leenen PJ, Themmen AP, van der Lely AJ (2013) Des-acyl ghrelin analogs prevent high-fat-diet-induced dysregulation of glucose homeostasis. FASEB J 27(4):1690–1700. https://doi.org/10.1096/fj.12-221143
Mao Y, Cheng J, Yu F, Li H, Guo C, Fan X (2015) Ghrelin attenuated lipotoxicity via autophagy induction and nuclear factor-kappab inhibition. Cell Physiol Biochem 37(2):563–576. https://doi.org/10.1159/000430377
Bischoff SC, Boirie Y, Cederholm T, Chourdakis M, Cuerda C, Delzenne NM, Deutz NE, Fouque D, Genton L, Gil C, Koletzko B, Leon-Sanz M, Shamir R, Singer J, Singer P, Stroebele-Benschop N, Thorell A, Weimann A, Barazzoni R (2017) Towards a multidisciplinary approach to understand and manage obesity and related diseases. Clin Nutr 36(4):917–938. https://doi.org/10.1016/j.clnu.2016.11.007
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
As this review does not provide original results, formal consent is not required.
Additional information
This article is part of the topical collection on Italian Society of Obesity’s Reviews.
Rights and permissions
About this article
Cite this article
Gortan Cappellari, G., Barazzoni, R. Ghrelin forms in the modulation of energy balance and metabolism. Eat Weight Disord 24, 997–1013 (2019). https://doi.org/10.1007/s40519-018-0599-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40519-018-0599-6