Abstract
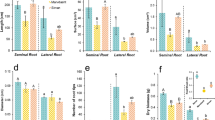
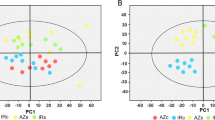
This study was undertaken to understand the root-driven differential response of enzymatic and non-enzymatic adaptive systems to drought in rice using 30 contrasting deep and shallow-rooted rice genotypes from the temperate rice diversity of Western Himalayan Kashmir under drought stress conditions. Our analysis focused on root and shoot traits and the assessment of 12 biochemical parameters encompassing both enzymatic and non-enzymatic responses. Under drought stress, deep-rooted genotypes exhibited significantly higher mean root depths, root biomass, and root-to-shoot ratios than their shallow-rooted counterparts. Interestingly, most of the biochemical parameters displayed an increasing trend in deep-rooted genotypes but decreased or showed non-significant increases in shallow-rooted genotypes under drought stress. Drought stress induced substantial changes in root and shoot parameters, with more pronounced effects observed in root traits. Deep-rooted genotypes demonstrated a remarkable 176.7% increase in root depth under drought conditions, compared to a modest 25.7% increase in irrigated conditions. Moreover, both enzymatic and non-enzymatic parameters exhibited higher increases in deep-rooted genotypes than shallow-rooted ones under drought stress. Our study unveiled significant associations among root and shoot traits and biochemical parameters, emphasizing the crucial role of roots in maintaining cellular homeostasis under stress conditions. These findings provide insights into the root-driven modulation of differential biochemical response of rice under drought stress, providing a foundation for further investigations into the molecular mechanisms through a multi-omics approach.

Similar content being viewed by others
References
Akram, N. A., Shafiq, F., & Ashraf, M. (2017). Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Frontiers in Plant Science, 8, 238088.
Al-Taweel, K., Iwaki, T., Yabuta, Y., Shigeoka, S., Murata, N., & Wadano, A. (2007). A bacterial transgene for catalase protects translation of D1 protein during exposure of salt-stressed tobacco leaves to strong light. Plant Physiology, 145(1), 258–265.
Alves, A. A., & Setter, T. L. (2004). Response of cassava leaf area expansion to water deficit: Cell proliferation, cell expansion and delayed development. Annals of Botany, 94(4), 605–613.
Aslam, M. M., Rashid, M. A. R., Siddiqui, M. A., Khan, M. T., Farhat, F., Yasmeen, S., & Yan, Z. (2022). Recent insights into signaling responses to cope drought stress in rice. Rice Science, 29(2), 105–117.
Bates, L. S., Waldren, R. A., & Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil, 39, 205–207.
Beauchamp, C., & Fridovich, I. (1971). Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Analytical Biochemistry, 44(1), 276–287.
Beena, R., Kirubakaran, S., Nithya, N., Manickavelu, A., Sah, R. P., Abida, P. S., & Siddique, K. H. (2021). Association mapping of drought tolerance and agronomic traits in rice (Oryza sativa L.) landraces. BMC Plant Biology, 21(1), 1–21.
Cvikrova, M., Gemperlova, L., Martincova, O., & Vankova, R. (2013). Effect of drought and combined drought and heat stress on polyamine metabolism in proline-over-producing tobacco plants. Plant Physiology and Biochemistry, 73, 7–15.
Das, K., & Roychoudhury, A. (2014). Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Frontiers in Environmental Science, 2, 53.
Farooq, M. A., Ali, S., Hameed, A., Bharwana, S. A., Rizwan, M., Ishaque, W., & Iqbal, Z. (2016). Cadmium stress in cotton seedlings: Physiological, photosynthesis and oxidative damages alleviated by glycinebetaine. South African Journal of Botany, 104, 61–68.
Fukai, S., & Cooper, M. (1995). Development of drought-resistant cultivars using physiomorphological traits in rice. Field Crops Research, 40(2), 67–86.
Gill, S. S., & Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry, 48(12), 909–930.
Hassan, M. A., Dahu, N., Hongning, T., Qian, Z., Yueming, Y., Yiru, L., & Shimei, W. (2023). Drought stress in rice: Morpho-physiological and molecular responses and marker-assisted breeding. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2023.1215371
Hodges, D. M., DeLong, J. M., Forney, C. F., & Prange, R. K. (1999). Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta, 207, 604–611.
Hsiao, T. C., & Xu, L. K. (2000). Sensitivity of growth of roots versus leaves to water stress: Biophysical analysis and relation to water transport. Journal of Experimental Botany, 51(350), 1595–1616.
Hussain, S., Khalid, M. F., Saqib, M., Ahmad, S., Zafar, W., Rao, M. J., & Anjum, M. A. (2018). Drought tolerance in citrus rootstocks is associated with better antioxidant defense mechanism. Acta Physiologiae Plantarum, 40, 1–10.
Impa, S. M., Nadaradjan, S. & Jagadish, S. V. K. (2012). Drought stress induced reactive oxygen species and anti-oxidants in plants. Abiotic Stress Responses in Plants: Metabolism, Productivity and Sustainability, pp. 131–147.
Jongdee, B., Fukai, S., & Cooper, M. (1998). Genotypic variation for grain yield of rice under water-deficit conditions. In: Proceedings of 9th Australian Agronomy Conference, Wagga Wagga. pp. 403–406.
Kaisangsri, N., Kowalski, R. J., Wijesekara, I., Kerdchoechuen, O., Laohakunjit, N., & Ganjyal, G. M. (2016). Carrot pomace enhances the expansion and nutritional quality of corn starch extrudates. LWT-Food Science and Technology, 68, 391–399.
Kang, J., Peng, Y., & Xu, W. (2022). Crop root responses to drought stress: Molecular mechanisms, nutrient regulations, and interactions with microorganisms in the rhizosphere. International Journal of Molecular Sciences, 23(16), 9310.
Kumar, S., Seem, K., & Mohapatra, T. (2023). Biochemical and epigenetic modulations under drought: Remembering the stress tolerance mechanism in rice. Life, 13(5), 1156.
Maehly, A. C. (1954). The assay of catalases and peroxidases. Methods Biochem Analysis, 1, 357–408. https://doi.org/10.1002/9780470110171.ch14
Marimuthu, R., Gurunathan, S., Sellamuthu, R., & Dhanarajan, A. (2023). Physio-biochemical characterizations in the drought induced rice (Oryza sativa L.): Pathway to understand the drought tolerance mechanisms. Plant Physiology Reports, 28(3), 388–404.
Menge, D. M., Kameoka, E., Kano-Nakata, M., Yamauchi, A., Asanuma, S., Asai, H., & Makihara, D. (2016). Drought-induced root plasticity of two upland NERICA varieties under conditions with contrasting soil depth characteristics. Plant Production Science, 19(3), 389–400.
Nahar, S., Vemireddy, L. R., Sahoo, L., & Tanti, B. (2018). Antioxidant protection mechanisms reveal significant response in drought-induced oxidative stress in some traditional rice of Assam, India. Rice Science, 25(4), 185–196.
Naikoo, M. I., Dar, M. I., Raghib, F., Jaleel, H., Ahmad, B., Raina, A., & Naushin, F. (2019). Role and regulation of plants phenolics in abiotic stress tolerance: An overview. Plant Signaling Molecules. https://doi.org/10.1016/B978-0-12-816451-8.00009-5
Naz, H. I. R. A., Akram, N. A., & Ashraf, M. (2016). Impact of ascorbic acid on growth and some physiological attributes of cucumber (Cucumis sativus) plants under water-deficit conditions. Pakistan Journal of Botany, 48(3), 877–883.
Nicotra, A. B., & Davidson, A. (2010). Adaptive phenotypic plasticity and plant water use. Functional Plant Biology, 37(2), 117–127.
Panda, D., Mishra, S. S., & Behera, P. K. (2021). Drought tolerance in rice: Focus on recent mechanisms and approaches. Rice Science, 28(2), 119–132.
Pandey, V., & Shukla, A. (2015). Acclimation and tolerance strategies of rice under drought stress. Rice Science, 22(4), 147–161.
Prieto, P., Pineda, M., & Aguilar, M. (1999). Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Analytical Biochemistry, 269(2), 337–341.
Priya, P., Patil, M., Pandey, P., Singh, A., Babu, V. S., & Senthil-Kumar, M. (2023). Stress combinations and their interactions in plants database: A one-stop resource on combined stress responses in plants. The Plant Journal, 116, 1097–1117.
Riemschneider, R., Abedin, M. Z., & Mocellin, R. P. (1976). Qualitats and stabilisierungprufung hitzekonservierter Nahrungsmittel unter verwendung von Vit C als kriterium-Mitt 1. Alimenta, 15, 171–171.
Sandhu, N., Raman, K. A., Torres, R. O., Audebert, A., Dardou, A., Kumar, A., & Henry, A. (2016). Rice root architectural plasticity traits and genetic regions for adaptability to variable cultivation and stress conditions. Plant Physiology, 171(4), 2562–2576.
Sandhu, N., Singh, A., Dixit, S., Sta Cruz, M. T., Maturan, P. C., Jain, R. K., & Kumar, A. (2014). Identification and mapping of stable QTL with main and epistasis effect on rice grain yield under upland drought stress. BMC Genetics, 15, 1–15.
Serraj, R. A. C. H. I. D., & Sinclair, T. R. (2002). Osmolyte accumulation: Can it really help increase crop yield under drought conditions? Plant, Cell and Environment, 25(2), 333–341.
Shafi, S., Shafi, I., Zaffar, A., Zargar, S. M., Shikari, A. B., Ranjan, A., & Sofi, P. A. (2023). The resilience of rice under water stress will be driven by better roots: Evidence from root phenotyping, physiological, and yield experiments. Plant Stress, 10, 100211.
Shikari, A. B., Najeeb, S., Khan, G., Mohidin, F. A., Shah, A. H., Nehvi, F. A., & Witcombe, J. R. (2021). KASP™ based markers reveal a population sub-structure in temperate rice (Oryza sativa L.) germplasm and local landraces grown in the Kashmir valley, north-western Himalayas. Genetic Resources and Crop Evolution, 68, 821–834.
Shukla, N., & Varma, Y. (2019). Enzymatic analysis of superoxide dismutase (SOD) from Hordeum vulgare: Its role in drought stress tolerance. Journal of Plant Biochemistry and Physiology, 7, 238.
Sibounheuang, V., Basnayake, J., & Fukai, S. (2006). Genotypic consistency in the expression of leaf water potential in rice (Oryza sativa L.). Field Crops Research, 97(2–3), 142–154.
Singh, A., Shamim, M. D., & Singh, K. N. (2013). Genotypic variation in root anatomy, starch accumulation, and protein induction in upland rice (Oryza sativa) varieties under water stress. Agricultural Research, 2, 24–30.
Singleton, V. L., Orthofer, R., & Lamuela-Raventós, R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In: Methods in enzymology Academic press. pp. 152–178.
Uga, Y., Okuno, K., & Yano, M. (2011). Dro1, a major QTL involved in deep rooting of rice under upland field conditions. Journal of Experimental Botany, 62(8), 2485–2494.
Uga, Y., Sugimoto, K., Ogawa, S., Rane, J., Ishitani, M., Hara, N., & Yano, M. (2013). Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nature Genetics, 45(9), 1097–1102.
Upadhyaya, H. & Panda, S. K. (2019). Drought stress responses and its management in rice. In: Advances in Rice Research for Abiotic Stress Tolerance, Woodhead Publishing. pp. 177–200.
Uzilday, B., Turkan, I., Sekmen, A. H., Ozgur, R. E. N. G., & Karakaya, H. C. (2012). Comparison of ROS formation and antioxidant enzymes in Cleome gynandra (C4) and Cleome spinosa (C3) under drought stress. Plant Science, 182, 59–70.
Vighi, I. L., Benitez, L. C., Amaral, M. N., Moraes, G. P., Auler, P. A., Rodrigues, G. S., & Braga, E. J. B. (2017). Functional characterization of the antioxidant enzymes in rice plants exposed to salinity stress. Biologia Plantarum, 61(3), 540–550.
Wade, L. J. (1999). Critical characteristics of rainfed rice environments and implications for rice improvement. In: Genetic Improvement of Rice for Water-limited Environments. International Rice Research Institute, Los Baños, Philippines, pp: 1–10.
Waterman, P. G., & Mole, S. (1994). Analysis of phenolic plant metabolites. Blackwell Scientific Publications.
Xu, W., Cui, K., Xu, A., Nie, L., Huang, J., & Peng, S. (2015). Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiologiae Plantarum, 37, 1–11.
Zhang, J. X., Nguyen, H. T., & Blum, A. (1999). Genetic analysis of osmotic adjustment in crop plants. Journal of Experimental Botany, 50, 291–302.
Zhang, Y., Luan, Q., Jiang, J., & Li, Y. (2021). Prediction and utilization of malondehyde in exotic pine under drought stress using near-infrared spectroscopy. Frontiers in Plant Sciences, 12, 735275.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shafi, S., Zaffar, A., Riyaz, I. et al. Differential drought responses in deep and shallow-rooted rice genotypes: enzymatic and non-enzymatic insights. Plant Physiol. Rep. (2024). https://doi.org/10.1007/s40502-024-00788-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40502-024-00788-2