Abstract
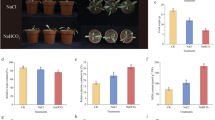
Safflower is an oilseed rabi crop. Continuous use of use of copper (Cu) containing fungicides and pesticides, due to high susceptibility of this crop of fungal attack has increased the accumulation of Cu in the soil. To investigate the effect of extra accumulated Cu on this particular crop, safflower seeds were germinated on different concentration of Cu (25, 50 and 100 µM) present in Hoagland’s solution (hydroponic) along with control (0.5 µM of Cu) and seedlings were harvested after 10 and 20 days of Cu treatment. The concentration of alpha-tocopherol was high in leaves as compared to roots in presence of Cu stress. The content of vitamin C was increased in both leaves and roots. In 10 and 20 day leaves, the CAT activity has been decreased in all the treatments. In 10 day roots, the CAT activity was increased but decreased abruptly in 20 day harvested roots. Isoenzymatic pattern of CAT showed three bands of CAT (CAT 1, CAT 2 and CAT 3) in control in 10 day leaves while CAT 1 was diminished in lane 2, 3 and 4. In 20 day leaves, we observed only one bands of CAT (CAT 2) in lane 1 (control) while CAT 1 was appeared at high concentration of Cu. We observed no more significant changes in the CAT isoforms in 10 and 20 day roots. The GPX activity was increased in both the day’s collected samples (leaves and roots). The analysis of GPX isoenzymes revealed no significant changes in isoenzymatic pattern but their band’s intensity was increased with an elevated metal treatment. In 20 day harvested leaves, we observed two bands of isoenzyme in lane 1 and four bands of GPX in lane 2, 3, 4 along with their increased intensities. In 10 day roots, we observed two band of GPX in all the treatment with elevated intensities as compared to control (lane 1). Similarly we observed two bands of GPX in all the treatments and control. But the intensity of bands was increased till lane 3 but in 20 day rootsat highest treatment (100 µM) the intensity of bands was decreased. The SOD activity was reduced in 10 and 20 day leaves. In 10 day roots, it was augmented but reduced at 100 µM as compared to control (100%). In 20 day harvested roots, the activity was decreased by 1.30, 11.11 and 15.68% with increased Cu concentration than control (100%). Three isoenzymes of SOD were appeared in 10 day leaves with more or less same intensity. While in 20 day leaves ex-plant has shown SOD 1, 2 and 3 activities. In 10 day treated roots SOD 1 with increasing intensity was appeared in lane 3 and 4 than control (lane 1) while SOD 2 was almost constantly present in all four lanes. Similarly in 20 day root ex-plant, SOD 1 has been observed with decreasing intensity in lane 2, 3 and 4 than control (lane 1). To cope up with the Cu stress, the antioxidant enzymes such as GPX and SOD were actively involved in safflower seedlings.













Similar content being viewed by others
Abbreviations
- ROS:
-
Reactive oxygen species
- CAT:
-
Catalase
- POD:
-
Peroxidase
- GPX:
-
Guaiacol peroxidase
- SOD:
-
Superoxide dismutase
- NBT:
-
Nitrobluetetrazolium
- SDS:
-
Sodium dodecyl sulfate
- FW:
-
Fresh weight
References
Adrees, M., Ali, S., Rizwan, M., Ibrahim, M., Abbas, F., Farid, M., et al. (2015). The effect of excess copper on growth and physiology of important food crops: A review. Environment Science and Pollution Research, 22, 8148–8162.
Ahmad, P., Jaleel, C. A., Azooz, M. M., & Nabi, G. (2009). Generation of ROS and non-enzymatic antioxidants during abiotic stress in plants. Botany Research International, 2(1), 11–20.
Al Chami, Z., Amer, N., Al Bitar, L., & Cavoski, I. (2015). Potential use of Sorghum bicolor and Carthamus tinctorius in phytoremediation of nickel, lead and zinc. International Journal of Environmental Science and Technology, 12(12), 3957–3970.
Alscher, R. G., Erturk, N., & Heath, L. S. (2002). Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. Journal of Experimental Botany, 53(372), 1331–1341.
Awasthy, J. M., Greeshma, G. M., Murukan, G., Krishan, V. G. M., & Murugan, K. (2015). Isozyme pattern of antioxidant enzymes and DNA damage in Octoblepharum albidum hedw. A bryophyte responses to cadmium and copper stress. Journal of Biological and Scientific Opinion, 3(1), 13–20.
Azeez, M. O., Adesanwo, O. O., & Adepetu, J. A. (2015). Effect of copper (Cu) application on soil available nutrients and uptake. African Journal of Agricultural Research, 10(5), 359–364.
Azooz, M. M., Abou-Elhamd, M. F., & Al-Fredan, M. A. (2012). Biphasic effect of copper on growth, proline, lipid peroxidation and antioxidant enzyme activities of wheat (Triticum aestivum cv. Hasaawi) at early growing stage. Australian Journal of Crop Science, 6(4), 688–694.
Bano, S., Ashraf, M., & Akram, N. A. (2014). Salt stress regulates enzymatic and nonenzymatic antioxidative defense system in the edible part of carrot (Daucus carota L.). Journal of Plant Interactions, 9(1), 324–329.
Beauchamp, C., & Fridovich, I. (1971). Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Analytical Biochemistry, 44(1), 276–287.
Benzie, I. F. F., & Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochemistry, 239(1), 70–76.
Bhaduri, A. M., & Fulekar, M. H. (2012). Antioxidant enzyme responses of plants to heavy metal stress. Reviews in Environmental Science & Biotechnology, 11, 55–69.
Brand-Williams, W., Cuvelier, M. E., & Berset, C. (1995). Use of a free radical method to evaluate the antioxidant activity. LWT—Food Science and Technology, 28(1), 25–30.
Cakmak, I., & Horst, W. (1991). Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase and peroxidase activities in root tip of soybean (glysin max). Journal of Plant Physiology, 83, 463–468.
Cosgrove, D. J. (1997). Assembly and enlargement of the primary cell wall in plants. Annual Review of Cell Biology, 13, 171–201.
Dhindsa, R. S., Plumb-Dhindsa, P., & Thorpe, T. A. (1980). Leaf senescence correlated with increased levels of membrane permeability and lipid-peroxidation and decreased levels of superoxide dismutase and catalase. Journal of Experimental Botany, 32(1), 93–101.
Filek, M., Keskinen, R., Hartikainen, H., Szarejko, I., Janiak, A., Miszalski, Z., et al. (2008). The protective role of selenium in rape seedlings subjected to cadmium stress. Plant Physiology, 165(8), 833–844.
Fridovich, I. (1986). Biological effects of superoxide radical. Archives of Biochemistry and Biophysics, 247, 1–11.
Gao, S., Yan, R., Cao, M., Yang, W., Wang, S., & Chen, F. (2008). Effects of copper on growth, antioxidant enzymes and phenylalanine ammonia-lyase activities in Jatropha curcas L. seedling. Plant Soil and Environment, 54(3), 117–122.
Gautam, S., Anjani, K., & Srivastava, N. (2016). In vitro evaluation of excess copper affecting seedlings and their biochemical characteristics in Carthamus tinctorius L. (variety PBNS-12). Physiology and Molecular Biology of Plants. https://doi.org/10.1007/s12298-016-0339-1.
Gautam, S., Bhagyawant, S. S., & Srivastava, N. (2014). Detailed study on therapeutic properties, uses and pharmacological applications of safflower (Carthamus tinctorius L.). International Journal of Ayurveda and Pharma Research, 2(3), 1–12.
Halliwell, B., & Gutteridge, J. M. C. (1999). Free radicals in biology and medicine (4th ed.). New York: Oxford University Press.
Hanumantharaya, L., Balikai, R. A., Mallapur, C. P., Venkateshalu., & Kumar, C. J. (2008). Integrated pest management strategies against safflower aphid, Uroleucon compositae (Theobald). In 7th International safflower conference, Wagga Wagga, Australia.
Jaishee, N., & Chakraborty, U. (2014). Evaluation of in-vitro antioxidant activities of Pteris biaurita L. International Journal of Pharmacy and Pharmaceutical Sciences, 6(2), 413–421.
Jaleel, C. A. (2009). Non-enzymatic antioxidant changes in Withania somnifera with varying drought stress levels. American-European Journal of Scientific Research, 4(2), 64–67.
Jayaraman, J. (1996). Laboratory manual in biochemistry fifth reprint. New Delhi: New Age International Ltd.
Khatun, S., Ali, M. B., Hahn, E. J., & Paek, K. Y. (2008). Copper toxicity in Withania somnifera: Growth and antioxidant enzymes responses of in vitro grown plants. Environmental and Experimental Botany, 64(3), 279–285.
Kochhar, S., Kochhar, V. K., & Khanduja, S. D. (1979). Changes in the pattern of isoperoxidases during maturation of grape berries cv gulabi as affected by ethephon phosphonic acid. American Journal of Enology and Viticulture, 30(4), 275–277.
Laemmli, U. K. (1970). Cleavage of structural proteins during assembly of head bacteriophage T4. Nature, 227(5259), 680–685.
Lagrimini, L. M. (1991). Wound-induced deposition of polyphenols in transgenic plants overexpressing peroxidase. Plant Physiology, 96, 577–583.
Li, Q., Li, Y., Li, C., & Yu, X. (2012). Enhanced ascorbic acid accumulation through overexpression of dehydroascorbate reductase confers tolerance to methyl viologen and salt stresses in tomato. Czech Journal of Genetics and Plant Breeding, 48, 74–86.
Li, S., Zhang, G., Gao, W., Zhao, X., Deng, C., & Lu, L. (2015). Plant growth, development and change in GSH level in safflower (Carthamus tinctorius L.) exposed to copper and lead. Archives of Biological Sciences, Belgrade, 67(2), 385–396.
Lin, C. C., & Kao, C. H. (2000). Effect of NaCl stress on H2O2 metabolism in rice leaves. Journal of Plant Growth Regultion, 30, 151–155.
Lombardi, L., & Sebastiani, L. (2005). Copper toxicity in Prunus cerasifera: Growth and antioxidant enzymes responses of in vitro grown plants. Plant Science, 168(3), 797–802.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the folin phenol reagent. The Journal of Biological Chemistry, 193, 265–275.
Malar, S., Vikram, S. S., Favas, P. J., & Perumal, V. (2014). Lead heavy metal toxicity induced changes on growth and antioxidative enzymes level in water hyacinths [Eichhornia crassipes (Mart.)]. Botanical Studies, 55, 54.
Mishra, S., Srivastava, S., Tripathi, R. D., Govindarajan, R., Kuriakose, S. V., & Prasad, M. N. V. (2006). Phytochelatin synthesis and response of antioxidants antioxidants during cadmium stress in Bacopa monnieri L. Plant Physiology and Biochemistry, 44(1), 24–37.
Munné-Bosch, S., & Alegre, L. (2002). The function of tocopherol and tocotrienols in plants. Critical Review in Plant Sciences, 21, 31–57.
Namdjoyan, S. H., Khavari-Nejad, R. A., Bernard, F., Nejadsattari, T., & Shaker, H. (2011). Antioxidant defense mechanisms in response to cadmium treatments in two safflower cultivars. Russian Journal of Plant Physiology, 58(3), 467–477.
Namdjoyan, S., Namdjoyan, S., & Kermanian, H. (2012). Induction of phytochelatin and responses of antioxidants under cadmium stress in safflower (Carthamus tinctorius) seedlings. Turkish Journal of Botany, 36, 495–502.
Nosrati, S., Asli, E. D., & Pazoki, A. (2013). Studying the resistance, absorption and accumulation of cadmium in safflower (Carthamus tinctorius L.) plant. Annals of Biological Research, 4, 169–173.
Polle, A., Otter, T., & Seifert, F. (1994). Apoplastic peroxidises and lignification in needles of Norway Spruce Picea abies L. Plant Physiology, 106(1), 53–60.
Pulido, R., Bravo, L., & Saura-Calixto, F. (2000). Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. Journal of Agricultural and Food Chemistry, 48(8), 402–3396.
Quiroga, M., Guerrero, C., Botella, A., Barcelo, M., Amaya, I., Medina, M. I., et al. (2000). A tomato peroxidase involved in the synthesis of lignin and suberin. Plant Physiology, 122, 1119–1127.
Rout, J. R., Ram, S. S., Das, R., Chakraborty, A., Sudarshan, M., & Sahoo, S. L. (2013). Copper-stress induced alternations in protein profile and antioxidant enzymes activities in the in vitro grown Withania somnifera L. Physiology and Molecular Biology of Plants, 19(3), 353–361.
Schützendübel, A., Schwanz, P., Teichmann, T., Gross, K., Langenfeld-Heyser, R., Godbold, D. L., et al. (2001). Cadmium-induced changes in antioxidative systems, H2O2 content and differentiation in pine (pinus sylvestris) roots. Plant Physiology, 127, 887–892.
Sharma, A., & Singh, G. (2013). Studies on the effect of Cu (II) ions on the antioxidant enzymes in chickpea (Cicer arietinum L) cultivars. Journal of Stress Physiology and Biochemistry, 9(1), 5–13.
Singh, N., Ma, L. Q., Srivastava, M., & Rathinasabapathi, B. (2006). Metabolic adaptations to arsenic-induced oxidative stress in Pteris vittata L. and Pteris ensiformis L. Plant Science, 170(2), 274–282.
Velich, I., Lakatos, S., Vegvarl, A., Stefanovlts-Banyal, E., & Sardi, E. (2000). Study of peroxidase isozyme pattern on susceptible bean genotypes natural infected with Pseudomonas syringae pv. savastanoi. Annual Report-Bean Improvement Cooperative, 43, 188–189.
Vitória, A. P., Lea, P. J., & Azevedo, R. A. (2001). Antioxidant enzymes responses to cadmium in radish tissues. Phytochemistry, 57, 701–710.
Wang, S. H., Yang, Z. M., Hong, Y., Lu, B., Li, S. Q., & Lu, Y. P. (2004). Copper-induced stress and antioxidative responses in roots of Brassica juncea L. Botanical Bulletin- Academia Sinica, 45, 203–212.
Willekens, H., Chamnongpol, S., Davey, M., Schraudner, M., Langebartels, C., Van Montagu, M., et al. (1997). Catalase is a sink for H2O2 and is indispensable for stress defense in C3 plants. European Molecular Biology Organization, 16, 4806–4816.
Woodbury, W., Spencer, A. K., & Stahmann, M. A. (1971). An improved procedure using ferricyanide for detecting catalase isozymes. Analytical Biochemistry, 44(1), 301–305.
Zengin, F. K., & Munzuroglu, O. (2005). Effects of some heavy metals on content of chlorophyll, proline and some antioxidant chemicals in bean (Phaseolus vulgaris L.) seedlings. Acta Biologica Cracoviensia Series: Botanica, 47(2), 157–164.
Acknowledgements
Authors would like to acknowledge Prof. Aditya Shastri, Vice Chancellor, Banasthali Vidyapith, for providing the facilities in the Department of Bioscience and Biotechnology for conducting the experiments. Also, we are grateful to Dr. K. Anjani, Principal Scientist, Directorate of Oilseed Research Institute, Hyderabad for providing the seeds to carry out the research study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gautam, S., Bhagyawant, S.S. & Srivastava, N. Antioxidant responses and isoenzyme activity of hydroponically grown safflower seedlings under copper stress. Ind J Plant Physiol. 23, 342–351 (2018). https://doi.org/10.1007/s40502-018-0378-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-018-0378-4