Abstract
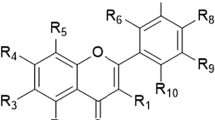
Polymethoxyflavones (PMFs) are one group of the flavonoid compounds, with tangeretin (Tan) and nobiletin (Nob) being the most abundant PMFs in citrus peel. Numerous biological activities of PMFs have been intensively studied, including anti-inflammatory and anticancer activities. Because of their methoxy groups, PMFs are more lipophilic than hydroxyl flavones, which may affect their biological activities. In addition, researchers found that hydroxylated PMFs (HPMFs) are one of the major metabolites of PMFs in animal urine and feces. Although PMF and HPMFs do show anticancer activity against different types of cancers, but their low hydrophilicity is still a crucial factor that may affect their biological effectiveness. Therefore, from the pharmaceutical aspect, chemical modifications of PMFs have been carried out to obtain acetylated PMFs (Ac-PMFs) for enhancing their biological effects. From the past centuries to the present, cancer is still a critical disease that needs to be solved. Carcinogenesis can be simply divided into three stages: initiation, promotion, and progression. These three stages involve different biological events, such as DNA mutation, cell proliferation, cell growth, and metastasis. In this paper, we aim to illustrate the biological effects of different PMFs, HPMFs, PMF derivatives, and metabolites against different types of cancer and related molecular mechanisms.



Similar content being viewed by others
Abbreviations
- ΔΨm:
-
mitochondrial membrane potential
- 5-Ac-Tan:
-
5-acetyl-6,7,8,4′-tetramethylnortangeretin
- 5-OH-HXMF:
-
5-hydorxy-3,6,7,8,3′,4′-hexamethoxyflavone
- 5-OH-Nob:
-
5-hydroxy-6,7,8,3′.4′-pcntamcthoxyflavanone
- 5-OH-PMFs:
-
5-hydroxylated PMFs
- 5-OH-Tan:
-
5-hydroxy-6,7,8,4′-tetramethoxyflavon
- AFB1:
-
aflatoxin B1γGT: γ-glutamyl transpeptidase
- BaP:
-
benzo[a]pyrene
- BM:
-
base-mentmembrane
- DFF-45:
-
DNA fragmentation factor
- DHTMF:
-
3,5-dihydroxy-6,7,3′,4′-tetramethoxyflavone
- ECM:
-
extracellular matrix
- GADD153:
-
enhanced DNA damage-inducible gene 153
- HPMFs:
-
hydroxylated polymethoxyflavone
- MMP:
-
matrix metalloproteinases
- Nob:
-
nobiletin
- PAH:
-
polycyclic aromatic hydrocarbons
- PARP:
-
poly(ADP-ribose) polymerase
- PMF:
-
polymethoxyflavone
- Sin:
-
sinensetin
- Tan:
-
tangeretin
References
Li S, Pan M-H, Lo C-Y, Tan D, Wang Y, Shahidi F, et al. Chemistry and health effects of polymethoxyflavones and hydroxylated polymethoxyflavones. J Funct Foods. 2009;1(1):2–12.
Li S, Lo C-Y, Ho C-T. Hydroxylated polymethoxyflavones and methylated flavonoids in sweet orange (Citrus sinensis) peel. J Agric Food Chem. 2006;54(12):4176–85.
Li S, Sang S, Pan M-H, Lai C-S, Lo C-Y, Yang CS, et al. Anti-inflammatory property of the urinary metabolites of nobiletin in mouse. Bioorg Med Chem Lett. 2007;17(18):5177–81.
Pan M-H, Chen W-J, Lin-Shiau S-Y, Ho C-T, Lin J-K. Tangeretin induces cell-cycle G1 arrest through inhibiting cyclin-dependent kinases 2 and 4 activities as well as elevating Cdk inhibitors p21 and p27 in human colorectal carcinoma cells. Carcinogenesis. 2002;23(10):1677–84.
Wang J, Duan Y, Zhi D, Li G, Wang L, Zhang H, et al. Pro-apoptotic effects of the novel tangeretin derivate 5-acetyl-6, 7, 8, 4′-tetramethylnortangeretin on mcf-7 breast cancer cells. Cell Biochem Biophys. 2014;70(2):1255–63.
Sudhakar A. History of cancer, ancient and modern treatment methods. J Cancer Sci Ther. 2009;1(2):1–4.
Organization WH. Cancer prevention 2018. Available from: https://www.who.int/cancer/prevention/en/. Accessed October 5 to November 9 in 2018
Blot WJ, Tarone RE. Doll and Peto’s quantitative estimates of cancer risks: holding generally true for 35 years. J Natl Cancer Inst. 2015;107(4):djv044.
Surh Y-J. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3(10):768–80.
Armitage P. Multistage models of carcinogenesis. Environ Health Perspect. 1985;63:195–201.
Pan M-H, Chiou Y-S, Wang Y-J, Ho C-T, Lin J-K. Multistage carcinogenesis process as molecular targets in cancer chemoprevention by epicatechin-3-gallate. Food Funct. 2011;2(2):101–10.
Lai C-S, Li S, Chai C-Y, Lo C-Y, Ho C-T, Wang Y-J, et al. Inhibitory effect of citrus 5-hydroxy-3, 6, 7, 8, 3′, 4′-hexamethoxyflavone on 12-O-tetradecanoylphorbol 13-acetate-induced skin inflammation and tumor promotion in mice. Carcinogenesis. 2007;28(12):2581–8.
Murakami A, Nakamura Y, Torikai K, Tanaka T, Koshiba T, Koshimizu K, et al. Inhibitory effect of citrus nobiletin on phorbol ester-induced skin inflammation, oxidative stress, and tumor promotion in mice. Cancer Res. 2000;60(18):5059–66.
Tang M, Ogawa K, Asamoto M, Hokaiwado N, Seeni A, Suzuki S, et al. Protective effects of citrus nobiletin and auraptene in transgenic rats developing adenocarcinoma of the prostate (TRAP) and human prostate carcinoma cells. Cancer Sci. 2007;98(4):471–7.
Wu JC, Tsai ML, Lai CS, Lo CY, Ho CT, Wang YJ, et al. Polymethoxyflavones prevent benzo [a] pyrene/dextran sodium sulfate-induced colorectal carcinogenesis through modulating xenobiotic metabolism and ameliorate autophagic defect in ICR mice. Int J Cancer. 2018;142(8):1689–701.
Lai C-S, Ho M-H, Tsai M-L, Li S, Badmaev V, Ho C-T, et al. Suppression of adipogenesis and obesity in high-fat induced mouse model by hydroxylated polymethoxyflavones. J Agric Food Chem. 2013;61(43):10320–8.
Tung Y-C, Li S, Huang Q, Hung W-L, Ho C-T, Wei G-J, et al. 5-Demethylnobiletin and 5-acetoxy-6, 7, 8, 3′, 4′-pentamethoxyflavone suppress lipid accumulation by activating the LKB1-AMPK pathway in 3T3-L1 preadipocytes and high fat diet-fed C57BL/6 mice. J Agric Food Chem. 2016;64(16):3196–205.
Knekt P, Kumpulainen J, Järvinen R, Rissanen H, Heliövaara M, Reunanen A, et al. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. 2002;76(3):560–8.
Gao Z, Gao W, Zeng S-L, Li P, Liu E-H. Chemical structures, bioactivities and molecular mechanisms of citrus polymethoxyflavones. J Funct Foods. 2018;40:498–509.
Nelson E. The occurrence of a pentamethyl flavonol in tangerine peel. J Am Chem Soc. 1934;56(6):1392–3.
Tseng K-F. 190. Nobiletin. Part I. J Chem Soc (Resumed). 1938:1003–4.
Kinoshita T, Firman K. Myricetin 5, 7, 3′, 4′, 5′-pentamethyl ether and other methylated flavonoids from Murraya paniculata. Phytochemistry. 1997;45(1):179–81.
Yenjai C, Prasanphen K, Daodee S, Wongpanich V, Kittakoop P. Bioactive flavonoids from Kaempferia parviflora. Fitoterapia. 2004;75(1):89–92.
Rajudin E, Ahmad F, Sirat HM, Arbain D, Aboul-Enein HY. Chemical constituents from tiger’s betel, Piper porphyrophyllum NE Br.(Fam. Piperaceae). Nat Prod Res. 2010;24(4):387–90.
Sastry G, Row L. Chemical investigation of citrus mitis Blanco—III: isolation of two new flavanones. Tetrahedron. 1961;15(1–4):111–4.
Li S, Wang H, Guo L, Zhao H, Ho C-T. Chemistry and bioactivity of nobiletin and its metabolites. J Funct Foods. 2014;6:2–10.
Crozier A, Del Rio D, Clifford MN. Bioavailability of dietary flavonoids and phenolic compounds. Mol Asp Med. 2010;31(6):446–67.
Karaś M, Jakubczyk A, Szymanowska U, Złotek U, Zielińska E. Digestion and bioavailability of bioactive phytochemicals. Int J Food Sci Technol. 2017;52(2):291–305.
Mena P, Llorach R. New frontiers on the metabolism, bioavailability and health effects of phenolic compounds. Multidisciplinary Digital Publishing Institute; 2017.
Nielsen S, Breinholt V, Justesen U, Cornett C, Dragsted L. In vitro biotransformation of flavonoids by rat liver microsomes. Xenobiotica. 1998;28(4):389–401.
Nielsen S, Breinholt V, Cornett C, Dragsted L. Biotransformation of the citrus flavone tangeretin in rats. Identification of metabolites with intact flavane nucleus. Food Chem Toxicol. 2000;38(9):739–46.
Li S, Wang Z, Sang S, Huang MT, Ho CT. Identification of nobiletin metabolites in mouse urine. Mol Nutr Food Res. 2006;50(3):291–9.
Zheng J, Bi J, Johnson D, Sun Y, Song M, Qiu P, et al. Analysis of 10 metabolites of polymethoxyflavones with high sensitivity by electrochemical detection in high-performance liquid chromatography. J Agric Food Chem. 2015;63(2):509–16.
Zheng J, Song M, Dong P, Qiu P, Guo S, Zhong Z, et al. Identification of novel bioactive metabolites of 5-demethylnobiletin in mice. Mol Nutr Food Res. 2013;57(11):1999–2007.
Li YR, Li S, Ho C-T, Chang Y-H, Tan K-T, Chung T-W, et al. Tangeretin derivative, 5-acetyloxy-6, 7, 8, 4′-tetramethoxyflavone induces G2/M arrest, apoptosis and autophagy in human non-small cell lung cancer cells in vitro and in vivo. Cancer Biol Ther. 2016;17(1):48–64.
Chiou Y-S, Sang S, Cheng K-H, Ho C-T, Wang Y-J, Pan M-H. Peracetylated (−)-epigallocatechin-3-gallate (AcEGCG) potently prevents skin carcinogenesis by suppressing the PKD1-dependent signaling pathway in CD34+ skin stem cells and skin tumors. Carcinogenesis. 2013;34(6):1315–22.
Rubio S, Quintana J, Eiroa JL, Triana J, Estévez F. Acetyl derivative of quercetin 3-methyl ether-induced cell death in human leukemia cells is amplified by the inhibition of ERK. Carcinogenesis. 2007;28(10):2105–13.
Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Järvinen T, et al. Prodrugs: design and clinical applications. Nat Rev Drug Discov. 2008;7(3):255–70.
Stella VJ, Nti-Addae KW. Prodrug strategies to overcome poor water solubility. Adv Drug Deliv Rev. 2007;59(7):677–94.
Li S, Pan M-H, Lai C-S, Lo C-Y, Dushenkov S, Ho C-T. Isolation and syntheses of polymethoxyflavones and hydroxylated polymethoxyflavones as inhibitors of HL-60 cell lines. Bioorg Med Chem. 2007;15(10):3381–9.
Su Z-Y, Shu L, Lee JH, Fuentes F, Wang H, Wu T-Y, Yu S, Kong Y-NT. Perspective on Nrf2, epigenomics and cancer stem cells in cancer chemoprevention using dietary phytochemicals and traditional Chinese medicines. Prog Chem. 2013;25(9)1526–43. https://doi.org/10.7536/PC130717.
Siddiqui IA, Sanna V, Ahmad N, Sechi M, Mukhtar H. Resveratrol nanoformulation for cancer prevention and therapy. Ann N Y Acad Sci. 2015;1348(1):20–31.
Fouad YA, Aanei C. Revisiting the hallmarks of cancer. Am J Cancer Res. 2017;7(5):1016–36.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Liu Y, Yin T, Feng Y, Cona MM, Huang G, Liu J, et al. Mammalian models of chemically induced primary malignancies exploitable for imaging-based preclinical theragnostic research. Quant Imaging Med Surg. 2015;5(5):708–29.
Siess MH, Bon AML, Canivenc-Lavier MC, Suschetet M. Mechanisms involved in the chemoprevention of flavonoids. Biofactors. 2000;12(1–4):193–9.
Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem. 2004;279(23):23847–50.
Wen X, Walle UK, Walle T. 5, 7-Dimethoxyflavone downregulates CYP1A1 expression and benzo [a] pyrene-induced DNA binding in Hep G2 cells. Carcinogenesis. 2005;26(4):803–9.
Wen X, Walle T. Preferential induction of CYP1B1 by benzo [a] pyrene in human oral epithelial cells: impact on DNA adduct formation and prevention by polyphenols. Carcinogenesis. 2005;26(10):1774–81.
Baird WM, Hooven LA, Mahadevan B. Carcinogenic polycyclic aromatic hydrocarbon-DNA adducts and mechanism of action. Environ Mol Mutagen. 2005;45(2–3):106–14.
Ma L-L, Wang D-w YX-D, Zhou Y-L. Tangeretin induces cell cycle arrest and apoptosis through upregulation of PTEN expression in glioma cells. Biomed Pharmacother. 2016;81:491–6.
Dong Y, Cao A, Shi J, Yin P, Wang L, Ji G, et al. Tangeretin, a citrus polymethoxyflavonoid, induces apoptosis of human gastric cancer AGS cells through extrinsic and intrinsic signaling pathways. Oncol Rep. 2014;31(4):1788–94.
Das A, Miller R, Lee P, Holden CA, Lindhorst SM, Jaboin J, et al. A novel component from citrus, ginger, and mushroom family exhibits antitumor activity on human meningioma cells through suppressing the Wnt/β-catenin signaling pathway. Tumor Biol. 2015;36(9):7027–34.
Zhu WB, Xiao N, Liu XJ. Dietary flavonoid tangeretin induces reprogramming of epithelial to mesenchymal transition in prostate cancer cells by targeting the PI3K/Akt/mTOR signaling pathway. Oncol Lett. 2018;15(1):433–40.
Surichan S, Arroo RR, Tsatsakis AM, Androutsopoulos VP. Tangeretin inhibits the proliferation of human breast cancer cells via CYP1A1/CYP1B1 enzyme induction and CYP1A1/CYP1B1–mediated metabolism to the product 4′ hydroxy tangeretin. Toxicol in Vitro. 2018;50:274–84.
Zhang X, Zheng L, Sun Y, Wang T, Wang B. Tangeretin enhances radiosensitivity and inhibits the radiation-induced epithelial-mesenchymal transition of gastric cancer cells. Oncol Rep. 2015;34(1):302–10.
Luo G, Guan X, Zhou L. Apoptotic effect of citrus fruit extract nobiletin on lung cancer cell line A549 in vitro and in vivo. Cancer Biol Ther. 2008;7(6):966–73.
Chen C, Ono M, Takeshima M, Nakano S. Antiproliferative and apoptosis-inducing activity of nobiletin against three subtypes of human breast cancer cell lines. Anticancer Res. 2014;34(4):1785–92.
Lien LM, Wang MJ, Chen RJ, Chiu HC, Wu JL, Shen MY, et al. Nobiletin, a polymethoxylated flavone, inhibits glioma cell growth and migration via arresting cell cycle and suppressing MAPK and Akt pathways. Phytother Res. 2016;30(2):214–21.
Sp N, Kang D, Kim D, Park J, Lee H, Kim H, et al. Nobiletin inhibits CD36-dependent tumor angiogenesis, migration, invasion, and sphere formation through the Cd36/Stat3/Nf-Κb signaling axis. Nutrients. 2018;10(6):772.
Cheng H-L, Hsieh M-J, Yang J-S, Lin C-W, Lue K-H, Lu K-H, et al. Nobiletin inhibits human osteosarcoma cells metastasis by blocking ERK and JNK-mediated MMPs expression. Oncotarget. 2016;7(23):35208–23.
Lee Y-C, Cheng T-H, Lee J-S, Chen J-H, Liao Y-C, Fong Y, et al. Nobiletin, a citrus flavonoid, suppresses invasion and migration involving FAK/PI3K/Akt and small GTPase signals in human gastric adenocarcinoma AGS cells. Mol Cell Biochem. 2011;347(1–2):103–15.
Baek SH, Kim S-M, Nam D, Lee J-H, Ahn KS, Choi S-H, et al. Antimetastatic effect of nobiletin through the down-regulation of CXC chemokine receptor type 4 and matrix metallopeptidase-9. Pharm Biol. 2012;50(10):1210–8.
Chen J, Chen AY, Huang H, Ye X, Rollyson WD, Perry HE, et al. The flavonoid nobiletin inhibits tumor growth and angiogenesis of ovarian cancers via the Akt pathway. Int J Oncol. 2015;46(6):2629–38.
Surichan S, Androutsopoulos VP, Sifakis S, Koutala E, Tsatsakis A, Arroo RR, et al. Bioactivation of the citrus flavonoid nobiletin by CYP1 enzymes in MCF7 breast adenocarcinoma cells. Food Chem Toxicol. 2012;50(9):3320–8.
Surichan S, Arroo RR, Ruparelia K, Tsatsakis AM, Androutsopoulos VP. Nobiletin bioactivation in MDA-MB-468 breast cancer cells by cytochrome P450 CYP1 enzymes. Food Chem Toxicol. 2018;113:228–35.
Chien S-Y, Hsieh M-J, Chen C-J, Yang S-F, Chen M-K. Nobiletin inhibits invasion and migration of human nasopharyngeal carcinoma cell lines by involving ERK1/2 and transcriptional inhibition of MMP-2. Expert Opin Ther Targets. 2015;19(3):307–20.
Androutsopoulos VP, Ruparelia K, Arroo RR, Tsatsakis AM, Spandidos DA. CYP1-mediated antiproliferative activity of dietary flavonoids in MDA-MB-468 breast cancer cells. Toxicology. 2009;264(3):162–70.
Qiu P, Dong P, Guan H, Li S, Ho CT, Pan MH, et al. Inhibitory effects of 5-hydroxy polymethoxyflavones on colon cancer cells. Mol Nutr Food Res. 2010;54(S2):S244–S52.
Chiou Y-S, Zheng Y-N, Tsai M-L, Lai C-S, Ho C-T, Pan M-H. 5-Demethylnobiletin more potently inhibits colon cancer cell growth than nobiletin in vitro and in vivo. JFB. 2018;2:91–7-–7.
Wu J-C, Tung Y-C, Zheng Y-N, Tsai M-L, Lai C-S, Ho C-T, et al. 5-Demethylnobiletin is more effective than nobiletin in preventing AOM/DSS-induced colorectal carcinogenesis in ICR mice. JFB. 2018;2:98–103-98.
Pan M-H, Lai Y-S, Lai C-S, Wang Y-J, Li S, Lo C-Y, et al. 5-hydroxy-3,6,7,8,3′,4′-hexamethoxyflavone induces apoptosis through reactive oxygen species production, growth arrest and DNA damage-inducible gene 153 expression, and caspase activation in human leukemia cells. J Agric Food Chem. 2007;55(13):5081–91.
Wang X, Xia M. 5-Hydroxy-3, 6, 7, 8, 3′, 4′-hexamethoxyflavone, a polymethoxyflavone, exerts antitumor effect on PI3K/Akt signaling pathway in human gastric cancer cell BGC-7901. J Recept Signal Transduction. 2016;36(5):471–7.
Cao C, Liu B, Zeng C, Lu Y, Chen S, Yang L, et al. A polymethoxyflavone from Laggera pterodonta induces apoptosis in imatinib-resistant K562R cells via activation of the intrinsic apoptosis pathway. Cancer Cell Int. 2014;14(1):137.
Blagosklonny MV, Pardee AB. The restriction point of the cell cycle. Cell Cycle. 2002;1(2):102–9.
Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int. 2014;2014:1–23.
Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516.
Li-Weber M. Targeting apoptosis pathways in cancer by Chinese medicine. Cancer Lett. 2013;332(2):304–12.
Hengartner MO. Apoptosis: DNA destroyers. Nature. 2001;412(6842):27–9.
Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(6805):770–6.
Steeg PS. Metastasis suppressors alter the signal transduction of cancer cells. Nat Rev Cancer. 2003;3(1):55–63.
Brooks SA, Lomax-Browne HJ, Carter TM, Kinch CE, Hall DM. Molecular interactions in cancer cell metastasis. Acta Histochem. 2010;112(1):3–25.
Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2(3):161–74.
Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9(4):239–52.
Rooprai HK, Kandanearatchi A, Maidment S, Christidou M, Trillo-Pazos G, Dexter DT, et al. Evaluation of the effects of swainsonine, captopril, tangeretin and nobiletin on the biological behaviour of brain tumour cells in vitro. Neuropathol Appl Neurobiol. 2001;27(1):29–39.
Androutsopoulos VP, Mahale S, Arroo RR, Potter G. Anticancer effects of the flavonoid diosmetin on cell cycle progression and proliferation of MDA-MB 468 breast cancer cells due to CYP1 activation. Oncol Rep. 2009;21(6):1525–8.
Androutsopoulos VP, Tsatsakis AM, Spandidos DA. Cytochrome P450 CYP1A1: wider roles in cancer progression and prevention. BMC Cancer. 2009;9(1):187.
Wu X, Song M, Wang M, Zheng J, Gao Z, Xu F, et al. Chemopreventive effects of nobiletin and its colonic metabolites on colon carcinogenesis. Mol Nutr Food Res. 2015;59(12):2383–94.
Ishii K, Tanaka S, Kagami K, Henmi K, Toyoda H, Kaise T, et al. Effects of naturally occurring polymethyoxyflavonoids on cell growth, p-glycoprotein function, cell cycle, and apoptosis of daunorubicin-resistant T lymphoblastoid leukemia cells. Cancer Investig. 2010;28(3):220–9.
Tan K-T, Li S, Li YR, Cheng S-L, Lin S-H, Tung Y-T. Synergistic anticancer effect of a combination of paclitaxel and 5-demethylnobiletin against lung cancer cell line in vitro and in vivo. Appl Biochem Biotechnol. 2018:1–16. https://doi.org/10.1007/s12010-018-2869-1.
Yuan H, Sun B, Gao F, Lan M. Synergistic anticancer effects of andrographolide and paclitaxel against A549 NSCLC cells. Pharm Biol. 2016;54(11):2629–35.
Arafa E-SA, Zhu Q, Barakat BM, Wani G, Zhao Q, El-Mahdy MA, et al. Tangeretin sensitizes cisplatin-resistant human ovarian cancer cells through downregulation of phosphoinositide 3-kinase/Akt signaling pathway. Cancer Res. 2009:0008–5472. CAN-09-1543.
Akao Y, Ohguchi K, Iinuma M, Nozawa Y. Interactive effects of polymethoxy flavones from Citrus on cell growth inhibition in human neuroblastoma SH-SY5Y cells. Bioorg Med Chem. 2008;16(6):2803–10.
Uesato S, Yamashita H, Maeda R, Hirata Y, Yamamoto M, Matsue S, et al. Synergistic antitumor effect of a combination of paclitaxel and carboplatin with nobiletin from Citrus depressa on non-small-cell lung cancer cell lines. Planta Med. 2014;80(06):452–7.
Funding
This study was financially supported by the Ministry of Science and Technology [105-2628-B-002-003-MY3, 107-2811-B-002-564].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Natural Products: From Chemistry to Pharmacology
Rights and permissions
About this article
Cite this article
Tung, YC., Chou, YC., Hung, WL. et al. Polymethoxyflavones: Chemistry and Molecular Mechanisms for Cancer Prevention and Treatment. Curr Pharmacol Rep 5, 98–113 (2019). https://doi.org/10.1007/s40495-019-00170-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40495-019-00170-z