Abstract
The presentation of allergic diseases in children with autism spectrum disorder (ASD) was evaluated systematically through a literature search using MEDLINE, EMBASE, Cochrane Library, and CINAHL databases. Any comparative studies on children with ASD and allergic diseases were evaluated for eligibility followed by risk of bias assessment, data synthesis, and meta-analysis. No randomized clinical trials were identified but 10 eligible observational studies were found, all of low methodological quality. A high estimated prevalence of asthma (OR 1.69, 95 % CI 1.11 to 2.59; 2,191 ASD children) and atopic rhinitis (OR 1.66, 95 % CI 1.49 to 1.85; 1,973 ASD children) were indicated. Rates of food allergy did not show significant differences between groups. Currently, clinical evidence was not found to draw any specific clinical implication.
Similar content being viewed by others
Introduction
Autism spectrum disorder (ASD) comprises a wide spectrum of developmental and functional impairment in the brain, and the prognosis varies depending on the symptom severity manifested in the individual (Fernell et al. 2013; Howlin et al. 2004; Levy and Perry 2011). ASD is characterized by a deficit of social interaction and personal skills, impairment in verbal and non-verbal communication, repetitive patterns of behavior, and notable consuming interests (American Psychiatric Association 2013). The diagnostic assessment for autism requires an elaborate screening process, which involves substantial consultations with many specialists and other physicians (Myers and Johnson 2007). The global prevalence of autism is estimated to be one in 160 people, and many studies have reported that the combination of genetic and environmental factors implicate a strong association in some aspects of ASD (Bailey et al. 1995; Campbell et al. 2006; Elsabbagh et al. 2012; Hallmayer et al. 2011); however, the development of ASD continues to be largely unclear.
Recently, a concern about the comorbidity of autism with allergy symptoms has been increasing along with rates of allergy among school children worldwide (Pawankar et al. 2013). Several studies have suggested a heightened prevalence of immune abnormalities and allergic diseases, including atopic dermatitis, asthma, allergic rhinitis, and food allergies, in children with ASD (Noriega and Savelkoul 2014; Jyonouchi 2010; Jung 2015; Altarac 2008). The collective opinions from these studies are difficult to define due to inconsistent findings on the association between ASD and allergic diseases; therefore, more perspective on evaluating these mixed findings are required. On the basis of these inconsistent reports, we speculate that children with ASD could be susceptible to specific types of allergic disease.
Allergic disease is caused by an acute immunological response that involves mast cells, basophils, and eosinophil activation by allergens cross-linking with immunoglobulin E (IgE) (Gould et al. 2003). This allergic cascade subsequently triggers the immediate hypersensitivity response with the release of histamine and other inflammatory mediators such as cytokines or the eosinophil response (Corry and Kheradmand 1999). Some studies have demonstrated that the imbalance between inflammatory mediators and their counter-regulatory molecules could affect the central nervous system homeostasis, which can result in neurological disorders or possible ASD development in the affected child (Wei et al. 2011; Goyal and Miyan 2014; Ashwood et al. 2006). Other studies have also proposed that allergy can induce stress, which could also indirectly instigate ASD development (Dave et al. 2011; Liezmann et al. 2011; Fine et al. 2014). Based on these implications, several evidence-based reviews have evaluated ASD core behavior interventions involving immune system modulation, but the evidence did not support the effectiveness of the interventions (Millward et al. 2008; Williams et al. 2013; Williams et al. 2012). Meanwhile, a review on food allergen avoidance trials showed some behavioral improvement in children with ASD (Neggers 2011).
In this context, evaluating the association between ASD and allergic diseases is necessary to clarify the conflicting findings and to uncover more precise potential risk or confounding factors. This systematic review thus attempts to examine the association between ASD and different allergic diseases in children by compiling and summarizing available evidence both qualitatively and quantitatively.
Methods
Search Strategy and Selection Criteria
The methods used to conduct this review were in accordance with the Cochrane Handbook for Systematic Reviews of Interventions and MOOSE guidelines (Higgins and Green 2011; Stroup et al. 2000). A comprehensive database search was conducted on May 17, 2014 using MEDLINE, EMBASE, the Cochrane Library and CINAHL, three prerequisite databases, and a topic-specific database. The search approach was to combine synonyms and related terms and identify pertinent English language articles using thesaurus search terms (Higgins and Green 2011). Terms used included “child development disorders, pervasive,” “attention deficit disorder,” “learning disorder,” “autism,” and “hypersensitivity,” with the application of the “explode” function to include all appropriate narrower terms (e.g., autism, asthma, etc.), in all potentially relevant combinations (see search strategy in Appendix Table 2). The reference list retrieved from the database searches was reviewed by five authors in consultation with experts in the field as needed. EndNote version X6 software (Thomson Reuters, New York, NY, USA) was used to store and manage the retrieved citations for screening.
All comparative study designs that at least incorporated a control group, such as randomized clinical trials, non-randomized trials or cohorts with a control group comparison, and case-control and cross-sectional studies, were reviewed for potential inclusion. For observational studies (e.g., cohort studies with a control group or case-control studies), we included in the review only those in which the ASD group and control group were identified within the same time period with a clear indication that no overlap between the two groups was permitted. Inclusion criteria were set in compliance with standard Cochrane review protocols and with reference to the Non-Randomised Studies Methods Group (NRSMG) of the Cochrane Collaboration (Higgins and Green 2011).
Regarding population of interest, studies that included children up to 18 years of age who were clinically diagnosed with ASD (including autistic disorder, Asperger’s syndrome, and pervasive developmental disorder not otherwise specified) and with an exposure of allergies or immune hypersensitivity were eligible for this review. The criteria for considering children with ASD in this review were developed from both the established Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) and the current DSM-V (American Psychiatric Association 2000, 2013). The primary study factors included common allergic symptoms in children indicated by clinical diagnosis criteria (e.g., atopic dermatitis, asthma, rhinitis, and food allergies). Immune reactivity response in relation to serum IgE level, eosinophil count level, autoinflammatory response, and immune-related biochemical marker expression were examined as secondary study factors.
On the other hand, studies in which participants were clinically diagnosed with a comorbidity in another category of developmental disorders (e.g., attention deficit hyperactivity disorder, specific learning disabilities) or with a neurological disorder such as epilepsy were excluded. Studies of animal models, systematic reviews, single case reports, and articles that did not provide relevant or original full data were also excluded from this review (see excluded studies in Appendix Table 3).
Selection of Studies and Data Assessment
Three authors in one group and two authors in another group independently screened all titles and abstracts of publications identified by the search in order to assess their eligibility. After identifying potential titles and abstracts from the search, two groups of authors ensured that judgments were reproducible by comparing the selection results. When authors disagreed on the inclusion of a study, all authors resolved the issue by discussion. Studies that did not meet the criteria were excluded from this initial stage. The primary focus was to select papers focusing on children with ASD without other developmental disorder comorbidities that might obscure the exposure of the study population. Two authors then obtained the full-text articles of identified eligible studies for independent assessment to decide which studies fulfilled the inclusion criteria. When studies referred to related, previously published protocols or studies, the referenced studies were assessed for criteria eligibility as well. Any disagreement at this stage was resolved by discussion between all reviewers based on their expert opinions, including referral to other literature sources if necessary. Two authors independently performed risk of bias assessment and data extraction for included studies using a modified data collection form recommended by the Cochrane Handbook (Higgins and Green 2011). To assess risk of bias for non-randomized studies or observational studies, the validated Risk of Bias Assessment Tool for Non-randomized Studies (RoBANs) was used (Kim et al. 2013). In addition, the Cochrane Risk of Bias Assessment Tool for Non-Randomized Studies of Interventions (ACROBAT-NRSI) guideline was used to support judgment of study quality (Sterne et al. 2014). Critical assessments were conducted using the domain-based evaluation form linked to the risk of bias tool. Each component was categorized as low risk, unclear risk, or high risk according to the RoBANs tool (Kim et al. 2013). The Cochrane Collaboration’s summary assessments of risk of bias were followed in reference to response options for an overall RoB judgment and consisted of the following: (1) low: low risk of bias for all key domains, (2) unclear: unclear risk of bias for one or more key domains, and (3) high: high risk of bias for one or more key domains. Overall risk of bias across studies consisted of the following: (1) overall low risk of bias across studies, where most data from studies was classified as low risk of bias for all major domains; (2) overall unclear risk of bias across studies, where most data from studies was classified as low or unclear risk of bias; and (3) overall high risk of bias across studies, where the proportion of data from studies at high risk of bias was enough to influence the interpretation of the results (Higgins and Green 2011). Study characteristics and results were entered into Review Manager (RevMan) 5.3 software for data synthesis (Cochrane 2014).
Data Synthesis and Analysis
Studies with similar design characteristics were combined for meta-analysis. When outcome measures data were amenable to synthesis, they were entered into RevMan 5.3 software for pair-wise comparison. The prevalence effect sizes between ASD and control groups were calculated in the form of odds ratios (OR) and 95 % confidence intervals. Unless otherwise indicated, a p value cut-off point of 0.05 indicated statistical significance. A random-effects model was used to yield the summary quantification of the pool effect across the studies by each outcome. If the included studies were diverse in methodology or estimated with a significant inconsistency, both random-effects and fixed-effect models were applied to observe the estimation effect trend. Statistical heterogeneity between trials was evaluated by the Chi2 statistic method with the significance set at p value <0.10 and I 2 tests were used to determine inconsistency for the combined studies. For continuous outcome data, the inverse-variance random effects method was used to summarize differences in the levels of biomarkers, while the standardized mean difference (SMD) and 95 % confidence interval with significance set at 0.05 were used to report the summary statistics. A sensitivity analysis was also conducted to examine the overall effect estimation and confidence interval variation by combining all initial included studies in the meta-analysis. The same meta-analysis was then repeated only for those studies that strongly complied with the inclusion criteria.
Results
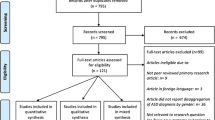
The search yielded 240 citations after duplications were removed. A flow diagram of included and excluded studies is shown in Fig. 1. All studies identified were observational comparative studies; high-quality study designs such as clinical randomized trials were not found to meet the objective of this review. After screening the titles and abstracts of studies initially identified as including an ASD population and allergy diseases, 16 articles were identified for full-text eligibility assessment. From the 16 selected articles, six studies were excluded based on reasons detailed in Appendix Table 3. A total of 10 studies were finally included in the meta-analysis. When meta-analysis was not possible for some outcome measures, available data were narratively described. Out of the 10 included studies, seven were case-control studies (Renzoni et al. 1995; Mrozek-Budzyn et al. 2013; Mostafa et al. 2008a, b, 2010; Magalhaes et al. 2009; Jyonouchi et al. 2008) and one was a cross-sectional study (Mostafa and Al-Ayadhi 2013). The remaining two were a population-based cross-sectional cohort study (Shibata et al. 2013) and a retrospective study (Chen et al. 2013). The included studies were conducted in the USA, Brazil, Egypt, Poland, Italy, Japan, and Taiwan.
A total of 10,380 children participated in the included studies, and 2,234 were children with ASD clinically diagnosed by DSM-III-R, DSM-IV, DSM-IV-TR, the Autism Diagnostic Interview-Revised (ADI-R), the Autism Diagnostic Observation Schedule (ADOS), or International Classification of Disease (ICD) code criteria. A summary of the characteristics of included studies is shown in Table 1. For reverse correlation comparison in population-based cross-sectional cohort studies, autistic traits were measured using the Japanese version of the Autism Screening Questionnaire (ASQ). Among the eligible studies, allergy diseases such as atopic dermatitis, asthma, allergic rhinitis, and food allergies, or intolerances were identified and categorized as the primary risk factors of interest. Autoimmune-related diseases such as ulcerative colitis, Crohn’s disease, type 1 diabetes, autoimmune thyroid disease, and Kawasaki disease were also found to be reported in some of the studies but were considered as secondary risk factors of interest in this review. These studies are presented in Appendix Table 4b.
Diagnoses of the allergy diseases in these included studies were based on clinical standardized diagnostic criteria using questionnaires, the ICD-9, or skin prick tests. Immune response measures were evaluated by clinical laboratory analysis of inflammatory cytokines, serum IgE, and eosinophil in children’s serum samples. Further details on measurements used and key results for all included studies are presented in Appendix Table 4b. These observational studies shared similar characteristics in terms of their objectives, methods, and participants, but the studies could not meet the quality equivalent of randomized trial studies. Overall, methodological quality of the included studies was assessed as almost uniformly low across all parameters based. Six studies were considered as unclear risk of bias (Chen et al. 2013; Magalhaes et al. 2009; Mostafa and Al-Ayadhi 2013; Mostafa et al. 2008b; Mrozek-Budzyn et al. 2013; Shibata et al. 2013) and four studies were of high risk of bias (Jyonouchi et al. 2008; Mostafa et al. 2008a, 2010; Renzoni et al. 1995). A table of risk of bias for each study is presented in Appendix Table 5a and a summary of overall risk of bias for outcomes across studies is presented in Appendix Table 5a.
Atopic Dermatitis
Five studies reported rates of atopic dermatitis symptoms in children with ASD compared with controls. Two were case-control studies (Mostafa et al. 2010; Jyonouchi et al. 2008), one was a cross-sectional study (Mostafa and Al-Ayadhi 2013), one was a population-based cross-sectional cohort study (Shibata et al. 2013), and one was a retrospective cohort study (Chen et al. 2013). The total number of participants for these four combined studies was 9,717, of whom 1,990 children were diagnosed with ASD (Fig. 2a). Meta-analysis of data from these studies showed a slight trend toward higher dermatitis rates in ASD compared to control groups (OR 1.30, 95 % CI 0.97 to 1.75; 1,990 ASD children, five studies, I 2 = 36 %), though the effect estimate did not reach a statistical significance (p = 0.08). There was no evidence of inconsistency or significant heterogeneity across the studies. After excluding studies with high risk of bias, the result did not show any significant change to the effect estimate (OR 1.28, 95 % CI 0.91 to 1.81, I 2 = 59 %, p = 0.15).
Asthma
Nine studies reported rates of asthma in children with ASD compared with controls. The studies comprised six case-control studies (Mrozek-Budzyn et al. 2013; Mostafa et al. 2008a, b, 2010; Magalhaes et al. 2009; Jyonouchi et al. 2008), one cross-sectional study (Mostafa and Al-Ayadhi 2013), one population-based cross-sectional cohort (Shibata et al. 2013), and one retrospective cohort study (Chen et al. 2013). The total number of participants from the nine studies was 10,215, of whom 2,191 children were identified as having ASD (Fig. 2b). The analysis showed that ASD children were more likely to have asthma compared to the control group (OR 1.69, 95 % CI 1.11 to 2.59; 2,191 ASD children, nine studies, I 2 = 49 %) with a statistical significance of (p = 0.02). Heterogeneity was, however, detected among these studies (p = 0.05). After eliminating studies with high risk of bias, the effect estimate did not differ significantly (OR 1.66, 95 % CI 1.12 to 2.46, I 2 = 41 %, p = 0.01).
Atopic Rhinitis
Of five studies reporting on atopic rhinitis rates in ASD children compared to controls, two were case-control studies (Magalhaes et al. 2009; Jyonouchi et al. 2008), one was a cross-sectional study (Mostafa and Al-Ayadhi 2013), one was a population-based cross-section cohort study (Shibata et al. 2013), and one was a retrospective cohort study (Chen et al. 2013). The total number of participants in the five studies was 9,685, of whom 1,973 children were identified as having ASD (Fig. 2c). Pooled analyses showed that children with ASD were more likely to have atopic rhinitis compared to their controls (OR 1.66, 95 % CI 1.49 to 1.85, 1,973 ASD children, five studies, I 2 = 0 %). The effect estimate showed a statistical significance of p < 0.00001 and there was no evidence of inconsistency and heterogeneity among the studies. Even after the high risk of bias study was excluded, the result did not show any significant change to the effect estimate (OR 1.68, 95 % CI 1.41 to 1.99, I 2 = 9 %, p < 0.00001).
Food Allergy Mediated by IgE
Three case-control studies reported on rates of IgE-mediated food allergy among ASD children compared with controls (Renzoni et al. 1995; Mrozek-Budzyn et al. 2013; Jyonouchi et al. 2008). The total number of participants from these studies was 550, of whom 272 children were identified as having ASD (Fig. 2d). The standard mean estimate odds ratio for food allergy in ASD children compared to controls did not imply statistical significance (p = 0.40), though there seemed a slightly higher susceptibility with ASD (OR 1.23, 95 % CI 0.76 to 2.01, 272 ASD children, three studies, I 2 = 0 %). No evidence of heterogeneity and inconsistency among the studies was detected. After excluding studies with high risk of bias, only one study was indicated (OR 1.25, 95 % CI 0.73 to 2.13, p = 0.41) and there was no significant difference in the overall effect estimate.
Total Serum IgE Level and Eosinophil Count
Three case-control studies reported on total serum IgE levels in children with ASD relative to those without (Renzoni et al. 1995; Mostafa et al. 2008b; Magalhaes et al. 2009). Since one of the three studies used a different standard of measurement, geometric mean (kU/L), and standardized mean (IU/L) in reporting the outcome (Renzoni et al. 1995), only two studies could be combined in the meta-analysis. The total number of participants from the two studies in the meta-analysis was 130, of whom 65 children were identified as having ASD (Fig. 3). Based on the pooled results, total serum IgE levels were higher in children with ASD relative to the control group (SMD 0.67, 95 % CI −0.03 to 1.36; 65 ASD children, two studies, I 2 = 65 %), but the difference did not reach a statistical significance (p = 0.06). Moderate heterogeneity was evident across the studies. Likewise, the study that was not included in the meta-analysis (Renzoni et al. 1995) did not show a statistically significant difference in the total serum IgE expression between children with ASD (geometric mean 66, 95 % CI 48 to 90 kU/L) and controls (geometric mean 65, 95 % CI 44 to 96 kU/L).
Forest plot of summary-standardized mean differences (SMD) of total serum IgE levels between the ASD group and control group. The original measurement unit for the total serum IgE in mean ± standard error was adjusted to mean and standard deviation for meta-analysis (IU/ml) (see Appendix Table 4b for original data units)
Additionally, two studies reported eosinophil counts in children with ASD relative to those without (Renzoni et al. 1995; Magalhaes et al. 2009). Both studies reported significantly higher eosinophil counts in the serum samples of the ASD group compared to the control group. One study (Magalhaes et al. 2009) with a total of 45 children, of whom 15 were specified as having Asperger’s syndrome, reported a higher geometric mean in eosinophil percentages in children with Asperger’s syndrome compared to that in the control group (p < 0.001). In contrast, the second study (Renzoni et al. 1995), in which 43 of 86 children had autism, reported that children with ASD had higher eosinophil absolute counts than controls (259.1 ± 27 vs. 193.4 ± 18 cells/cmm; p < 0.05).
Discussion
In this review, significantly elevated rates of both asthma (p = 0.02) and allergic rhinitis (p < 0.00001) were reported in children with ASD relative to those without. Specifically, children with ASD were 69 % more likely to have asthma than were those in the control groups, and, similarly, allergic rhinitis was 66 % more common in the ASD group than in the control group. One explanation for such high rates of allergic diseases in the ASD group could relate to immune sensitivity corresponding to intrinsic stress factors and/or psychosocial stress commonly found in children with ASD (Watling et al. 2001; Scifo et al. 1996; Liezmann et al. 2011; Fine et al. 2014; Corbett et al. 2009). In addition, some research has suggested that stress could aid in releasing neurogenic inflammatory agents, which is also identified in bronchial mucosa or immune cells, and conversely, that the immune system could also modulate the central nervous system function via various molecules, including cytokines (Fine et al. 2014; Dave et al. 2011; Akhondzadeh and Asadabadi 2012). Based on these speculations, the bidirectional mechanism process could also possibly account for the findings of the population-based cross-sectional cohort study carried out by Shibata et al. (2013), in which children with asthma or rhinitis had higher scores on the Japanese version of the ASQ than controlsthe children in the control group. The meta-analysis for the combined studies did not reflect the diagnostic period of ASD; however, findings supported the presence of the association between ASD and allergic diseases, particularly asthma and allergic rhinitis. Thus, additional research in determining this association during early detection periods of ASD is needed. This review did not, however, find significantly different rates of atopic dermatitis in the ASD group relative to controls, thereby implying that children with ASD could be more susceptible to a particular type of allergy rather than allergies in general.
Findings from this review did not support a significant association between ASD and IgE-mediated food allergy. The slightly higher rates in the ASD group than in the control group could be reflected by the high risk of bias of studies and the fact that allergen-specific IgE levels could change in association with the ability to tolerate food which attribute to false results from allergen testing (Panel 2010). As for the total serum IgE levels in children with ASD, the difference did not reach a statistical significance for the two combined studies, even though serum IgE level appeared higher in the ASD group. The natural history or typical progression of allergic diseases that begins early in a child’s life and the tendency for spontaneous remission of allergic diseases with age, known as “allergic march,” could also play a role in the association (Wahn 2000). However, more studies are necessary to clarify the differences between food allergies and food intolerance, as food intolerance is not the result of immediate allergic reactivity (Panel 2010). The meta-analysis result for total serum IgE levels was an expression of the pool estimate for SMD rather than the original units of measurement; therefore, more studies are required to investigate substantial difference estimates (Higgins and Green 2011).
Taken from the studies together, higher eosinophil counts were reported in children with ASD compared to the control group, which could be linked to the fact that production of IgE is regulated by a specific type of cytokine produced from Th2 cells, and the regulation process subsequently induces eosinophil activation (Woszczek et al. 2002; Sannohe et al. 2003; Busse 1996). In addition, Chen et al. (2013) identified Crohn’s disease (OR 1.44, 95 % CI 0.89 to 2.33) and type 1 diabetes (OR 4.01, 95 % CI 1.00 to 16.04) as having associations with ASD, which is also consistent with higher odds of an abnormal inflammatory response (see Appendix Table 4b for the summary of outcome measure results). Imbalances in proinflammatory markers like interleukin-23 (IL-23) or T-cell regulator CD4 cells, anti-myelin basic protein, and serotonin were also reported to be associated with ASD in the reviewed studies, but pooling of data for these immune symptoms was not possible due to the involvement of various cytokine and inflammatory response pathways. Other research suggests that an immune response and inflammation of the central nervous system could contribute to the pathogenesis of autism, which supports a possible mechanism for these observed associations (Wei et al. 2011; Ashwood et al. 2006). A consistent pattern of high rates of immune dysregulation is evident in children with ASD, although the differences in reporting across studies limited further data synthesis of this point.
A key strength of this review is its examination of rates of different types of allergic diseases in children with ASD by using a standardized estimate for consistent comparison. The findings from this review could facilitate in the advancement of coherent design and study for the comorbidity of ASD and allergic diseases, as well as intervention development. In referencing to the current DSM-5 diagnostic criteria, which has encompassed separate autistic disorders into a single diagnostic category, the inclusion criteria of participants were more consistent and refined. In addition, the manual also included diagnostic criteria for developmental disorders comorbidities such as attention-deficit/hyperactivity disorder, which in recent years also gained attention for high prevalence of allergies (Marshall 1989; Moffitt and Melchior 2007). This could set an opportunity for elucidating the specific subset of children with the comorbidity of ASD and other developmental disorders in association with allergic diseases in the future.
It is important to mention that all studies identified in the present review were observational studies, which are prone to unclear risk of bias in the selection of participants and blinding of outcome assessments across studies and yielded low overall quality of evidence. In each study, the most common drawback was small sample size and the inability to infer causal relationships between the exposure and outcome variable. Data retrieved from population-based cross-sectional studies or national databases provided preliminary association estimates; however, most cases of ASD could not be identified in a neutral setting, which may lead to a potentially skewed population due to the selection and diagnosis process. Another limitation of this systematic review is the different diagnostic criteria used in each study (e.g., ADOS, DSM-III, DSM-IV-TR, DSM-5, or ICD-10) such as the DSM-IV-TR diagnostic criteria, which did not include comorbidities of other developmental disorders as opposed to the current version, the DSM-5 diagnostic criteria. However, the core clinical concepts of ASD and ADHD have not been changed in the ADOS, DSM-IV-TR, DSM-5, or ICD-10, and hence, this current evaluation would unlikely be influenced by differences in criteria or versions (Worley and Matson 2012; Volkmar et al. 1992). Considering this limitation, the population of children with ASD is relatively sparse compared to the general population; therefore, the findings should be interpreted with caution in light of the risk of over-estimating the true power of significance from possible subtypes of the ASD population. As for allergic diseases, the optimal estimate for each type of allergic disease was limited by the different outcome measurements used among the studies. This could be partly due to the prevalence of allergens in the environment resulting in allergy testing variations in different countries. Future studies should consider these confounders and include a larger number of participants to strengthen statistical power. Publication bias was not investigated due to variations in study design and population size across studies. Given the lack of randomized controlled trials from the literature search at this time, there is a high possibility of confounding factors, such as genetic status of children, psychological stress, medication status, or comorbidity of different types of developmental disorders or allergic disease that could not be ruled out. Psychological stress in children could possibly be one of the key confounding factors between ASD and allergy although further investigations and more detailed analyses, such as meta-regressions, are needed.
The findings presented in this review only allowed for prevalence-based conclusions to be drawn from the available evidence. There is no clinical base of evidence at this point to support an etiological association between autism and allergy; however, children with ASD posed a high risk of specific asthma and atopic rhinitis, but not food allergy.
References
Akhondzadeh, S., & Asadabadi, M. (2012). Risperidone plus celecoxib in children with autistic disorder: a double-blind, randomized trial. British Journal of Clinical Pharmacology, 73(6), 983–984.
Altarac, M. (2008). Prevalence of allergies in US children with autism. Annals of Epidemiology, 18(9), 725.
American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders: DSM-IV-TR (4th ed.). Washington, DC: Author.
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Author.
Ashwood, P., Wills, S., & Van de Water, J. (2006). The immune response in autism: a new frontier for autism research. Journal of Leukocyte Biology, 80(1), 1–15.
Bailey, A., Le Couteur, A., Gottesman, I., Bolton, P., Simonoff, E., Yuzda, E., et al. (1995). Autism as a strongly genetic disorder: evidence from a British twin study. Psychological Medicine, 25(01), 63–77.
Busse, W. (1996). The role of leukotrienes in asthma and allergic rhinitis. Clinical and Experimental Allergy, 26(8), 868–879.
Campbell, D. B., Sutcliffe, J. S., Ebert, P. J., Militerni, R., Bravaccio, C., Trillo, S., et al. (2006). A genetic variant that disrupts MET transcription is associated with autism. Proceedings of the National Academy of Sciences, 103(45), 16834–16839.
Chen, M. H., Su, T. P., Chen, Y. S., Hsu, J. W., Huang, K. L., Chang, W. H., et al. (2013). Comorbidity of allergic and autoimmune diseases in patients with autism spectrum disorder: a nationwide population-based study. Research in Autism Spectrum Disorders, 7(2), 205–212.
Cochrane Collaboration (2014). Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration.
Corbett, B. A., Schupp, C. W., Levine, S., & Mendoza, S. (2009). Comparing cortisol, stress, and sensory sensitivity in children with autism. Autism Research, 2(1), 39–49.
Corry, D. B., & Kheradmand, F. (1999). Induction and regulation of the IgE response. Nature, 402(6760 Suppl), B18–23.
Dave, N. D., Xiang, L., Rehm, K. E., & Marshall, G. D., Jr. (2011). Stress and allergic diseases. Immunology and Allergy Clinics of North America, 31(1), 55–68.
Elsabbagh, M., Divan, G., Koh, Y. J., Kim, Y. S., Kauchali, S., Marcín, C., et al. (2012). Global prevalence of autism and other pervasive developmental disorders. Autism Research, 5(3), 160–179.
Fernell, E., Eriksson, M. A., & Gillberg, C. (2013). Early diagnosis of autism and impact on prognosis: a narrative review. Clinical Epidemiology, 5, 33.
Fine, R., Zhang, J., & Stevens, H. (2014). Prenatal stress and inhibitory neuron systems: implications for neuropsychiatric disorders. Molecular Psychiatry, 19(6), 641–651.
Gould, H. J., Sutton, B. J., Beavil, A. J., Beavil, R. L., McCloskey, N., Coker, H. A., et al. (2003). The biology of IgE and the basis of allergic disease. Annual Review of Immunology, 21(1), 579–628.
Goyal, D. K., & Miyan, J. A. (2014). Neuro-immune abnormalities in autism and their relationship with the environment: a variable insult model for autism. Front Endocrinol (Lausanne), 5, 29. doi:10.3389/fendo.2014.00029.
Hallmayer, J., Cleveland, S., Torres, A., Phillips, J., Cohen, B., Torigoe, T., et al. (2011). Genetic heritability and shared environmental factors among twin pairs with autism. Archives of General Psychiatry, 68(11), 1095–1102.
Higgins, J., & Green, S. E. (2011). Cochrane handbook for systematic reviews of interventions version 5.1.0. Chicherster: Wiley.
Howlin, P., Goode, S., Hutton, J., & Rutter, M. (2004). Adult outcome for children with autism. Journal of Child Psychology and Psychiatry, 45(2), 212–229.
Jung, J. H. (2015). The effect of PM10 on allergy symptoms in allergic rhinitis patients during spring season. World Allergy Organization Journal, 8(Suppl 1), A35.
Jyonouchi, H. (2010). Autism spectrum disorders and allergy: observation from a pediatric allergy/immunology clinic. [Research Support, Non-U.S. Gov’t]. Expert Review Clinical Immunology, 6(3), 397–411. doi:10.1586/eci.10.18.
Jyonouchi, H., Geng, L., Cushing-Ruby, A., & Quraishi, H. (2008). Impact of innate immunity in a subset of children with autism spectrum disorders: a case control study. Journal of Neuroinflammation, 5, 52. doi:10.1186/1742-2094-5-52.
Kim, S. Y., Park, J. E., Lee, Y. J., Seo, H. J., Sheen, S. S., Hahn, S., et al. (2013). Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. Journal of Clinical Epidemiology, 66(4), 408–414. doi:10.1016/J.Jclinepi.2012.09.016.
Levy, A., & Perry, A. (2011). Outcomes in adolescents and adults with autism: a review of the literature. Research in Autism Spectrum Disorders, 5(4), 1271–1282.
Liezmann, C., Klapp, B., & Peters, E. M. (2011). Stress, atopy and allergy: a re-evaluation from a psychoneuroimmunologic persepective. Dermato-endocrinology, 3(1), 37.
Magalhaes, E. S., Pinto-Mariz, F., Bastos-Pinto, S., Pontes, A. T., Prado, E. A., & deAzevedo, L. C. (2009). Immune allergic response in Asperger syndrome. Journal of Neuroimmunology, 216(1–2), 108–112. doi:10.1016/j.jneuroim.2009.09.015.
Marshall, P. (1989). Attention deficit disorder and allergy: a neurochemical model of the relation between the illnesses. Psychological Bulletin, 106(3), 434.
Millward, C., Ferriter, M., Calver, S., Connell-Jones, G. (2008). Gluten-and casein-free diets for autistic spectrum disorder. Cochrane Database Systematic Reviews, Issue 2, Art. No CD003498, Issue 2, Art. No. CD003498.
Moffitt, T. E., & Melchior, M. (2007). Why does the worldwide prevalence of childhood attention deficit hyperactivity disorder matter? The American Journal of Psychiatry, 164(6), 856–858.
Mostafa, G. A., & Al-Ayadhi, L. Y. (2013). The possible relationship between allergic manifestations and elevated serum levels of brain specific auto-antibodies in autistic children. Journal of Neuroimmunology, 261(1–2), 77–81. doi:10.1016/j.jneuroim.2013.04.003.
Mostafa, G. A., El-Sherif, D. F., Hamza, R. T., & Al Shehab, A. (2008a). Hyperserotonemia in Egyptian autistic children: relation to allergic manifestations. Journal of Pediatric Neurology, 6(3), 227–236.
Mostafa, G. A., Hamza, R. T., & El-Shahawi, H. H. (2008b). Allergic manifestations in autistic children: relation to disease severity. Journal of Pediatric Neurology, 6(2), 115–123.
Mostafa, G. A., Al Shehab, A., & Fouad, N. R. (2010). Frequency of CD4+CD25high regulatory T cells in the peripheral blood of Egyptian children with autism. Journal of Child Neurology, 25(3), 328–335. doi:10.1177/0883073809339393.
Mrozek-Budzyn, D., Majewska, R., Kielyka, A., & Augustyniak, M. (2013). The frequency and risk factors of allergy and asthma in children with autism—case-control study [comparative study]. Przegla̧d Epidemiologiczny, 67(4), 675–679. 761–674.
Myers, S. M., & Johnson, C. P. (2007). Management of children with autism spectrum disorders. Pediatrics, 120(5), 1162–1182.
Neggers, Y. (2011). Autism spectrum disorders—from genes to environment. T. Williams (Ed.), Dietary interventions in autism (http://www.intechopen.com/books/autism-spectrum-disorders-from-genes-to-environment/dietary-interventions-in-autism). Rijeka, InTech.
Noriega, D. B., & Savelkoul, H. F. (2014). Immune dysregulation in autism spectrum disorder. European Journal of Pediatrics, 173(1), 33–43.
Panel, N.-S. E. (2010). Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. Journal of Allergy and Clinical Immunology, 126(6), S1–S58.
Pawankar, R., Canonica, G., ST Holgate, S., Lockey, R., Blaiss, M. (2013). The WAO White Book on Allergy, Update 2013. Milwaukee: World Allergy Organization. Available at: http://www.worldallergy.org. Accessed 30 July 2014
Renzoni, E., Beltrami, V., Sestini, P., Pompella, A., Menchetti, G., & Zappella, M. (1995). Brief report: allergological evaluation of children with autism. Journal of Autism and Developmental Disorders, 25(3), 327–333.
Sannohe, S., Adachi, T., Hamada, K., Honda, K., Yamada, Y., Saito, N., et al. (2003). Upregulated response to chemokines in oxidative metabolism of eosinophils in asthma and allergic rhinitis. European Respiratory Journal, 21(6), 925–931.
Scifo, R., Cioni, M., Nicolosi, A., Batticane, N., Tirolo, C., Testa, N., et al. (1996). Opioid-immune interactions in autism: behavioural and immunological assessment during a double-blind treatment with naltrexone. Annali dell Istituto Superiore di Sanita, 32(3), 351–359.
Shibata, A., Hitomi, Y., Kambayashi, Y., Hibino, Y., Yamazaki, M., Mitoma, J., et al. (2013). Epidemiological study on the involvements of environmental factors and allergy in child mental health using the autism screening questionnaire. Research in Autism Spectrum Disorders, 7(1), 132–140.
Sterne, J. A. C., Higgins, J. P. T., Reeves, B. C. (2014). A Cochrane risk of bias assessment tool: for non-randomized studies of interventions (ACROBAT-NRSI). Version 1.0.0, 24 September 2014. The Cochrane Collaboration. Available from http://www.riskofbias.info. Accessed 20 December 2014.
Stroup, D. F., Berlin, J. A., Morton, S. C., Olkin, I., Williamson, G. D., Rennie, D., et al. (2000). Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA, 283(15), 2008–2012.
Volkmar, F. R., Cicchetti, D. V., Bregman, J., & Cohen, D. J. (1992). Three diagnostic systems for autism: DSM-III, DSM-III-R, and ICD-10. Journal of Autism and Developmental Disorders, 22(4), 483–492.
Wahn, U. (2000). What drives the allergic march? Allergy, 55(7), 591–599.
Watling, R. L., Deitz, J., & White, O. (2001). Comparison of sensory profile scores of young children with and without autism spectrum disorders. American Journal of Occupational Therapy, 55(4), 416–423.
Wei, H., Zou, H., Sheikh, A. M., Malik, M., Dobkin, C., Brown, W. T., et al. (2011). IL-6 is increased in the cerebellum of autistic brain and alters neural cell adhesion, migration and synaptic formation. Journal of Neuroinflammation, 8, 52. doi:10.1186/1742-2094-8-52.
Williams, K., Wray, J. A., Wheeler, D. M. (2012). Intravenous secretin for autism spectrum disorders (ASD). Cochrane Database Systematic Reviews, Issue 4, Art.No CD003495.
Williams, K., Wheeler, D., Silove, N., Hazell, P. (2013). Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews, Issue 8, Art. No. CD004677.
Worley, J. A., & Matson, J. L. (2012). Comparing symptoms of autism spectrum disorders using the current DSM-IV-TR diagnostic criteria and the proposed DSM-V diagnostic criteria. Research in Autism Spectrum Disorders, 6(2), 965–970.
Woszczek, G., Kowalski, M., & Borowiec, M. (2002). Association of asthma and total IgE levels with human leucocyte antigen-DR in patients with grass allergy. European Respiratory Journal, 20(1), 79–85.
Compliance with Ethical Standards
ᅟ
Funding
All phases of this study were supported by a National Center for Child Health and Development Grant (26A-5) and a Health Labour Sciences Research Grant (No. 13800128) from the Ministry of Health, Labour, and Welfare, Japan. None of these sources participated in any part of the performance of the study.
Conflict of Interest
The authors declare that they have no competing interests.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Miyazaki, C., Koyama, M., Ota, E. et al. Allergies in Children with Autism Spectrum Disorder: a Systematic Review and Meta-analysis. Rev J Autism Dev Disord 2, 374–401 (2015). https://doi.org/10.1007/s40489-015-0059-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40489-015-0059-4