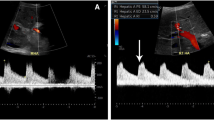
Abstract
Background and aims
There is limited literature on endoscopic ultrasound-guided liver biopsy (EUS-LB), a new method of obtaining liver biopsy (LB).
Methods
We conducted a retrospective study of the efficacy and safety of EUS-LB compared to percutaneous liver biopsy (PC-LB) in patients with chronic liver disease at our center between January 2018 and August 2019.
Results
Thirty patients underwent EUS-LB and 60 patients underwent PC-LB were identified (median follow-up post-LB was 8 days; interquartile range (IQR), 3–5 days). The median number of portal tracts was significantly higher in the PC-LB group (13 vs. 5; P < 0.0001). A histologic diagnosis was established in 93% of the EUS-LB group, compared to 100% in the PC-LB group (P = 0.841). Patients in EUS-LB group had significantly shorter hospital stay (median time of hospital stay was 3 vs. 4.2 h in the EUS-LB vs. PC-LB group, respectively; P = 0.004) and reported less pain compared to PC-LB group (median pain score was 0 vs. 3.5; P = 0.0009). EUS-LB were performed using a 19-gauge (n = 27) or 22-gauge (n = 3); there was a tendency towards higher number of portal tracts in the 22- vs. the 19-gauge needle group (6 vs. 5; P = 0.501). No patient in either group had significant adverse events such as bleeding or death.
Conclusion
EUS-LB is safe and is associated with less pain, shorter hospital stay, and high diagnostic yield (93%) compared to PC-LB. Randomized trials are needed to standardize the utility of EUS-LB.




Similar content being viewed by others
Abbreviations
- CHC:
-
Chronic hepatitis C
- CRN:
-
Clinical Research Network
- EH:
-
Eosinophilic hepatitis
- ESLD:
-
End-stage liver disease
- EUS-LB:
-
Endoscopic ultrasound-guided liver biopsy
- EUS-FNA/FNB:
-
Endoscopic ultrasound-guided fine needle aspiration or biopsy
- FLD:
-
Fatty liver disease
- IRB:
-
Institutional Review Board
- LB:
-
Liver biopsy
- NAFLD:
-
Non-alcoholic fatty liver disease
- NASH:
-
Non-alcoholic steatohepatitis
- NAS:
-
NAFLD activity score
- PBC:
-
Primary biliary cholangitis
- PC-LB:
-
Percutaneous-guided liver biopsy
- PFIC:
-
Progressive familial intrahepatic cholestasis
- VCTE:
-
Vibration-controlled transient elastography
References
Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM (2007) Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol 5(10):1214–1220
Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F et al (2003) Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol 29(12):1705–1713
Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD (2009) American Association for the Study of Liver D. Liver biopsy. Hepatology 49(3):1017–1044
Strassburg CP, Manns MP (2006) Approaches to liver biopsy techniques–revisited. Sem Liver Dis 26(4):318–327
Bravo AA, Sheth SG, Chopra S (2001) Liver biopsy. N Engl J Med 344(7):495–500
Diehl DL, Johal AS, Khara HS, Stavropoulos SN, Al-Haddad M, Ramesh J et al (2015) Endoscopic ultrasound-guided liver biopsy: a multicenter experience. Endosc Int Open 3(3):E210–E215
Yavuz K, Geyik S, Barton RE, Petersen B, Lakin P, Keller FS et al (2007) Transjugular liver biopsy via the left internal jugular vein. J Vasc Interv Radiol 18(2):237–241
Mok SRS, Diehl DL (2019) The role of EUS in liver biopsy. Curr Gastroenterol Rep 21(2):6
Mok SRS, Diehl DL, Johal AS, Khara HS, Confer BD, Mudireddy PR et al (2019) Endoscopic ultrasound-guided biopsy in chronic liver disease: a randomized comparison of 19-G FNA and 22-G FNB needles. Endosc Int Open 7(1):E62–E71
Pineda JJ, Diehl DL, Miao CL, Johal AS, Khara HS, Bhanushali A et al (2016) EUS-guided liver biopsy provides diagnostic samples comparable with those via the percutaneous or transjugular route. Gastrointest Endosc 83(2):360–365
Smith EH (1991) Complications of percutaneous abdominal fine-needle biopsy. Review. Radiology 178(1):253–258
Myers RP, Fong A, Shaheen AA (2008) Utilization rates, complications and costs of percutaneous liver biopsy: a population-based study including 4275 biopsies. Liver Int 28(5):705–712
Johal AS, Khara HS, Maksimak MG, Diehl DL (2014) Endoscopic ultrasound-guided liver biopsy in pediatric patients. Endosc Ultrasound 3(3):191–194
Parekh PJ, Majithia R, Diehl DL, Baron TH (2015) Endoscopic ultrasound-guided liver biopsy. Endosc Ultrasound 4(2):85–91
Shruti MSI, Vyas N, Sofi A, Das A (2018) Eus guided liver biopsy is more cost-effective than percutaneous liver biopsy in patients with non-alcoholic fatty liver disease (NAFLD). Gastrointest Endosc AB326–AB7
Sey MS, Al-Haddad M, Imperiale TF, McGreevy K, Lin J, DeWitt JM (2016) EUS-guided liver biopsy for parenchymal disease: a comparison of diagnostic yield between two core biopsy needles. Gastrointest Endosc 83(2):347–352
Nieto J, Khaleel H, Challita Y, Jimenez M, Baron TH, Walters L et al (2018) EUS-guided fine-needle core liver biopsy sampling using a novel 19-gauge needle with modified 1-pass, 1 actuation wet suction technique. Gastrointest Endosc 87(2):469–475
Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW et al (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41(6):1313–1321
Krebs EE, Carey TS, Weinberger M (2007) Accuracy of the pain numeric rating scale as a screening test in primary care. J Gen Intern Med 22(10):1453–1458
Demetris AJ, Ruppert K (2003) Pathologist's perspective on liver needle biopsy size? J Hepatol 39(2):275–277
Crawford AR, Lin XZ, Crawford JM (1998) The normal adult human liver biopsy: a quantitative reference standard. Hepatology 28(2):323–331
Bazerbachi F, Vargas EJ, Matar R, Storm AC, Mounajjed TM, Topazian MD et al (2019) EUS-guided core liver biopsy sampling using a 22-gauge fork-tip needle: a prospective blinded trial for histologic and lipidomic evaluation in nonalcoholic fatty liver disease. Gastrointest Endosc 90(6):926–932
Bjørn M, Brendstrup C, Karlsen S, Carlsen JE (1998) Consecutive screening and enrollment in clinical trials: the way to representative patient samples? J Cardiac Fail 4(3):225–230
Firpi RJ, Soldevila-Pico C, Abdelmalek MF, Morelli G, Judah J, Nelson DR (2005) Short recovery time after percutaneous liver biopsy: should we change our current practices? Clin Gastroenterol Hepatol 3(9):926–929
Friedman LS (2004) Controversies in liver biopsy: who, where, when, how, why? Curr Gastroenterol Rep 6(1):30–36
Grant A, Neuberger J (1999) Guidelines on the use of liver biopsy in clinical practice. British Society of Gastroenterology. Gut 45(Suppl 4):iv1–iv11.
Lindor KD, Bru C, Jorgensen RA, Rakela J, Bordas JM, Gross JB et al (1996) The role of ultrasonography and automatic-needle biopsy in outpatient percutaneous liver biopsy. Hepatology 23(5):1079–1083
Takyar V, Etzion O, Heller T, Kleiner DE, Rotman Y, Ghany MG et al (2017) Complications of percutaneous liver biopsy with Klatskin needles: a 36-year single-centre experience. Aliment Pharmacol Therap 45(5):744–753
Tublin ME, Blair R, Martin J, Malik S, Ruppert K, Demetris A (2017) Prospective study of the impact of liver biopsy core size on specimen adequacy and procedural complications. Am J Roentgenol 210(1):183–188
Vijayaraghavan GR, David S, Bermudez-Allende M, Sarwat H (2011) Imaging-guided parenchymal liver biopsy: how we do it. J Clin Imaging Sci 1:30
Mohan BP, Shakhatreh M, Garg R, Ponnada S, Adler DG (2019) Efficacy and safety of EUS-guided liver biopsy: a systematic review and meta-analysis. Gastrointest Endosc 89(2):238–46.e3
Colloredo G, Guido M, Sonzogni A, Leandro G (2003) Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol 39(2):239–244
Author information
Authors and Affiliations
Contributions
GMH, AHA, and SP contributed to the concept, design, literature search, clinical studies, data acquisition, statistical analysis, manuscript preparation, manuscript editing, and manuscript review. DSR and YG re-examined the liver biopsies. AA, SS, and JAI reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This is a retrospective study that involved only chart review. No animals were involved in any aspect of this study.
Informed consent
Obtaining informed consent has been waived by the institution’s IRB due to nature of the study (retrospective and chart review study).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ali, A.H., Panchal, S., Rao, D.S. et al. The efficacy and safety of endoscopic ultrasound-guided liver biopsy versus percutaneous liver biopsy in patients with chronic liver disease: a retrospective single-center study. J Ultrasound 23, 157–167 (2020). https://doi.org/10.1007/s40477-020-00436-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40477-020-00436-z