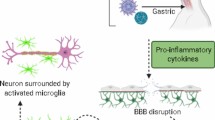
Abstract
Purpose of Review
West Nile virus (WNV) emerged from Central Africa in the 1990s and is now endemic throughout much of the world. Twenty years after its introduction in the USA, it is becoming apparent that neurological impairments can persist for years following infection. Here, we review the epidemiological data in support of such long-term deficits and discuss possible mechanisms that drive these persistent manifestations.
Recent Findings
Focusing on the recently discovered antimicrobial roles of amyloid and alpha-synuclein, we connect WNV late pathology to overlapping features encountered in neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease. We also summarize new research on microglial activation and engulfment of neural synapses seen in recovered WNV as well as in neurodegenerative diseases, and discuss how loss of integrity of the blood-brain barrier (BBB) may exacerbate this process.
Summary
Neuroinvasive viral infections such as WNV may be linked epidemiologically and mechanistically to neurodegeneration. This may open doors to therapeutic options for hitherto untreatable infectious sequelae; additionally, it may also shed light on the possible infectious etiologies of age-progressive neurodegenerative dementias.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Chancey C, Grinev A, Volkova E, Rios M. The global ecology and epidemiology of West Nile virus. Biomed Res Int. 2015;2015:376230.
Petersen LR, Carson PJ, Biggerstaff BJ, Custer B, Borchardt SM, Busch MP. Estimated cumulative incidence of West Nile virus infection in US adults, 1999-2010. Epidemiol Infect. 2013;141(3):591–5.
Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. JAMA. 2013;310(3):308–15.
Murray KO, Garcia MN, Rahbar MH, Martinez D, Khuwaja SA, Arafat RR, et al. Survival analysis, long-term outcomes, and percentage of recovery up to 8 years post-infection among the Houston West Nile virus cohort. PLoS One. 2014;9(7):e102953.
Carson PJ, Konewko P, Wold KS, Mariani P, Goli S, Bergloff P, et al. Long-term clinical and neuropsychological outcomes of West Nile virus infection. Clin Infect Dis. 2006;43(6):723–30.
Heneka MT, Golenbock DT, Latz E. Innate immunity in Alzheimer’s disease. Nat Immunol. 2015;16(3):229–36.
Sejvar JJ, Haddad MB, Tierney BC, Campbell GL, Marfin AA, Van Gerpen JA, et al. Neurologic manifestations and outcome of West Nile virus infection. JAMA. 2003;290(4):511–5.
Klee AL, Maidin B, Edwin B, Poshni I, Mostashari F, Fine A, et al. Long-term prognosis for clinical West Nile virus infection. Emerg Infect Dis. 2004;10(8):1405–11.
Ou AC, Ratard RC. One-year sequelae in patients with West Nile Virus encephalitis and meningitis in Louisiana. J La State Med Soc. 2005;157(1):42–6.
Watson JT, Pertel PE, Jones RC, Siston AM, Paul WS, Austin CC, et al. Clinical characteristics and functional outcomes of West Nile fever. Ann Intern Med. 2004;141(5):360–5.
Samaan Z, McDermid Vaz S, Bawor M, Potter TH, Eskandarian S, Loeb M. Neuropsychological impact of West Nile virus infection: an extensive neuropsychiatric assessment of 49 cases in Canada. PLoS One. 2016;11(6):e0158364.
Kathleen Y, Haaland JS, Pergam S, Echevarria LA, Davis LE, Goade D, et al. Mental status after West Nile virus infection. Emerg Infect Dis. 2006;12(8):1260–2.
Sadek JR, Pergam SA, Harrington JA, Echevarria LA, Davis LE, Goade D, et al. Persistent neuropsychological impairment associated with West Nile virus infection. J Clin Exp Neuropsychol. 2010;32(1):81–7.
•• Murray KO, Nolan MS, Ronca SE, Datta S, Govindarajan K, Narayana PA, et al. The neurocognitive and MRI outcomes of West Nile Virus infection: preliminary analysis using an external control group. Front Neurol. 2018;9:111 This study examines neuropsychological testing and MRI findings in patients an average of 4 years after their initial illness onset. Normative controls are used as a comparison group for the MRI portion of the study. The authors report significant frontal, temporal, and limbic cortical thinning in the WNV participants.
Sejvar JJ, Curns AT, Welburg L, Jones JF, Lundgren LM, Capuron L, et al. Neurocognitive and functional outcomes in persons recovering from West Nile virus illness. J Neuropsychol. 2008;2(Pt 2):477–99.
Sheppard DP, Woods SP, Hasbun R, Salazar L, Nolan MS, Murray KO. Does intra-individual neurocognitive variability relate to neuroinvasive disease and quality of life in West Nile Virus? J Neuro-Oncol. 2018;24(4):506–13.
Balakrishnan A, Thekkekara RJ, Tandale BV. Outcomes of West Nile encephalitis patients after 1 year of West Nile encephalitis outbreak in Kerala, India: a follow-up study. J Med Virol. 2016;88(11):1856–61.
Fray PJ, Robbins TW. CANTAB battery: proposed utility in neurotoxicology. Neurotoxicol Teratol. 1996;18(4):499–504.
Rabadi MH. Brought down by a mosquito? West Nile Virus encephalitis. Am J Med. 2018;131(9):1064–6.
Pradhan S, Anand S, Choudhury SS. Cognitive behavioural impairment with irreversible sensorineural deafness as a complication of West Nile encephalitis. J Neurovirol. 2019;25(3):429–33.
Lyons JL, Schaefer PW, Cho TA, Azar MM. Case 34-2017. A 76-year-old man with fever, weight loss, and weakness. N Engl J Med. 2017;377(19):1878–86.
Guth JC, Futterer SA, Hijaz TA, Liotta EM, Rosenberg NF, Naidech AM, et al. Pearls & oy-sters: bilateral thalamic involvement in West Nile virus encephalitis. Neurology. 2014;83(2):e16–7.
Brilla R, Block M, Geremia G, Wichter M. Clinical and neuroradiologic features of 39 consecutive cases of West Nile Virus meningoencephalitis. J Neurol Sci. 2004;220(1–2):37–40.
Racsa L, Gander R, Chung W, Southern P, Le J, Beal S, et al. Clinical features of West Nile virus epidemic in Dallas, Texas, 2012. Diagn Microbiol Infect Dis. 2014;78(2):132–6.
Ocal M, onder H, Arsava EM, Alp S, Ozkul A, Ergünay K. A case of central nervous system infection due to West Nile Virus lineage-1 in Ankara province, Turkey. Mikrobiyol Bul. 2013;47(1):164–72.
Mainali S, Afshani M, Wood JB, Levin MC. The natural history of West Nile virus infection presenting with West Nile virus meningoencephalitis in a man with a prolonged illness: a case report. J Med Case Rep. 2011;5:204.
Abraham A, Ziv S, Drory VE. Posterior reversible encephalopathy syndrome resulting from Guillain-Barre-like syndrome secondary to West Nile virus infection. J Clin Neuromuscul Dis. 2011;12(3):113–7.
Jain N, Fisk D, Sotir M, Kehl KS. West Nile encephalitis, status epilepticus and West Nile pneumonia in a renal transplant patient. Transpl Int. 2007;20(9):800–3.
Bosanko CM, Gilroy J, Wang AM, Sanders W, Dulai M, Wilson J, et al. West Nile Virus encephalitis involving the substantia nigra: neuroimaging and pathologic findings with literature review. Arch Neurol. 2003;60(10):1448–52.
DeBiasi RL, Parsons JA, Grabert BE. West Nile virus meningoencephalitis in an immunocompetent adolescent. Pediatr Neurol. 2005;33(3):217–9.
Ali M, Safriel Y, Sohi J, Llave A, Weathers S. West Nile virus infection: MR imaging findings in the nervous system. AJNR Am J Neuroradiol. 2005;26(2):289–97.
Rosas H, Wippold FJ 2nd. West Nile virus: case report with MR imaging findings. AJNR Am J Neuroradiol. 2003;24(7):1376–8.
Brener ZZ, et al. Acute renal failure in a patient with West Nile viral encephalitis. Nephrol Dial Transplant. 2007;22(2):662–3.
Shepherd JC, Subramanian A, Montgomery RA, Samaniego MD, Gong G, Bergmann A, et al. West Nile virus encephalitis in a kidney transplant recipient. Am J Transplant. 2004;4(5):830–3.
Farnaes L, Schiff D, McElroy A, Coufal NG, Crawford JR, Cannavino C. Encephalitis and thalamic injury from neuroinvasive West Nile Virus in children on treatment for acute lymphoblastic leukemia. Pediatr Neurol. 2018;80:84–7.
Petropoulou KA, Gordon SM, Prayson RA, Ruggierri PM. West Nile virus meningoencephalitis: MR imaging findings. AJNR Am J Neuroradiol. 2005;26(8):1986–95.
Hwang J, Ryu H-S, Kim H, Lee S-A. The first reported case ofWest Nile encephalitis in Korea. J Korean Med Sci. 2015;30(3):343–5.
Kadkhoda K, Embil JM, McKibbin LR, McEachern J, Michael A. Drebot West Nile Virus infection in a renal transplant recipient resulting in polioencephalomylelitis, quadriplegia, and global brain atrophy. IDCases. 2019;17:e00551.
Ledig C, Schuh A, Guerrero R, Heckemann RA, Rueckert D. Structural brain imaging in Alzheimer’s disease and mild cognitive impairment: biomarker analysis and shared morphometry database. Sci Rep. 2018;8(1):11258.
Helmich RC, Vaillancourt DE, Brooks DJ. The future of brain imaging in Parkinson’s disease. J Park Dis. 2018;8(s1):S47–51.
Verma S, Lo Y, Chapagain M, Lum S, Kumar M, Gurjav U, et al. West Nile virus infection modulates human brain microvascular endothelial cells tight junction proteins and cell adhesion molecules: transmigration across the in vitro blood-brain barrier. Virology. 2009;385(2):425–33.
Diamond MS, Shrestha B, Mehlhop E, Sitati E, Engle M. Innate and adaptive immune responses determine protection against disseminated infection by West Nile encephalitis virus. Viral Immunol. 2003;16(3):259–78.
Samuel MA, Wang H, Siddharthan V, Morrey JD, Diamond MS. Axonal transport mediates West Nile virus entry into the central nervous system and induces acute flaccid paralysis. Proc Natl Acad Sci U S A. 2007;104(43):17140–5.
Shrestha B, Gottlieb D, Diamond MS. Infection and injury of neurons by West Nile encephalitis virus. J Virol. 2003;77(24):13203–13.
Hayes EB, Sejvar JJ, Zaki SR, Lanciotti RS, Bode AV, Campbell GL. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg Infect Dis. 2005;11(8):1174–9.
Diniz JA, da Rosa AP, Guzman H, Xu F, Xiao SY, Popov VL, et al. West Nile virus infection of primary mouse neuronal and neuroglial cells: the role of astrocytes in chronic infection. Am J Trop Med Hyg. 2006;75(4):691–6.
Lesteberg KE, Beckham JD. Immunology of West Nile virus infection and the role of alpha-synuclein as a viral restriction factor. Viral Immunol. 2019;32(1):38–47.
Suthar MS, Diamond MS, Gale M Jr. West Nile virus infection and immunity. Nat Rev Microbiol. 2013;11(2):115–28.
Appler KK, Brown AN, Stewart BS, Behr MJ, Demarest VL, Wong SJ, et al. Persistence of West Nile virus in the central nervous system and periphery of mice. PLoS One. 2010;5(5):e10649.
Stewart BS, Demarest VL, Wong SJ, Green S, Bernard KA. Persistence of virus-specific immune responses in the central nervous system of mice after West Nile virus infection. BMC Immunol. 2011;12:6.
Matthews KA, Xu W, Gaglioti AH, Holt JB, Croft JB, Mack D, et al. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015-2060) in adults aged >/=65 years. Alzheimers Dement. 2019;15(1):17–24.
Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71(5):362–81.
Selkoe DJ. The molecular pathology of Alzheimer’s disease. Neuron. 1991;6(4):487–98.
Doody RS, Farlow M, Aisen PS, Alzheimer’s Disease Cooperative Study Data Analysis and Publication Committee. Phase 3 trials of solanezumab and bapineuzumab for Alzheimer’s disease. N Engl J Med. 2014;370(15):1460.
Doody RS, Raman R, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. Alzheimer's Disease Cooperative Study Steering Committee, Siemers E, Sethuraman G, Mohs R; Semagacestat Study Group. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N Engl J Med. 2013;369(4):341–50.
Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370(4):311–21.
LaMotte S. Another promising Alzheimer’s drug trial ends in failure: This one hurts: CNN Health; 2019.
Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73(24):2061–70.
Gosztyla ML, Brothers HM, Robinson SR. Alzheimer’s amyloid-beta is an antimicrobial peptide: a review of the evidence. J Alzheimers Dis. 2018;62(4):1495–506.
Moir RD, Lathe R, Tanzi RE. The antimicrobial protection hypothesis of Alzheimer’s disease. Alzheimers Dement. 2018;14(12):1602–14.
Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B, et al. The Alzheimer’s disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS One. 2010;5(3):e9505.
Kumar DK, Choi SH, Washicosky KJ, Eimer WA, Tucker S, Ghofrani J, et al. Moir RD Amyloid-beta peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci Transl Med. 2016;8(340):340–72.
Bourgade K, Dupuis G, Frost EH, Fülöp T. Anti-viral properties of amyloid-beta peptides. J Alzheimers Dis. 2016;54(3):859–78.
Bourgade K, Le Page A, Bocti C, Witkowski JM, Dupuis G, Frost EH, et al. Protective effect of amyloid-beta peptides against herpes simplex virus-1 infection in a neuronal cell culture model. J Alzheimers Dis. 2016;50(4):1227–41.
Eimer WA, Vijaya Kumar DK, Navalpur Shanmugam NK, Rodriguez AS, Mitchell T, Washicosky KJ, et al. Alzheimer’s disease-associated beta-amyloid is rapidly seeded by herpesviridae to protect against brain infection. Neuron. 2018;100(6):1527–32.
Chai Q, Jovasevic V, Malikov V, Sabo Y, Morham S, Walsh D, et al. HIV-1 counteracts an innate restriction by amyloid precursor protein resulting in neurodegeneration. Nat Commun. 2017;8(1):1522.
Ezzat K, Pernemalm M, Pålsson S, Roberts TC, Järver P, Dondalska A, et al. The viral protein corona directs viral pathogenesis and amyloid aggregation. Nat Commun. 2019;10(1):2331.
White MR, Kandel R, Tripathi S, Condon D, Qi L, Taubenberger J, et al. Alzheimer’s associated beta-amyloid protein inhibits influenza A virus and modulates viral interactions with phagocytes. PLoS One. 2014;9(7):e101364.
Park SC, Moon JC, Shin SY, Son H, Jung YJ, Kim NH, et al. Functional characterization of alpha-synuclein protein with antimicrobial activity. Biochem Biophys Res Commun. 2016;478(2):924–8.
•• Beatman EL, Massey A, Shives KD, Burrack KS, Chamanian M, Morrison TE, et al. Alpha-synuclein expression restricts RNA viral infections in the brain. J Virol. 2015;90(6):2767–82 This murine study shows that neuronal alpha-synuclein inhibited WNV infection in the CNS, and that deletion of the gene for alpha-synuclein gene resulted in decreased survival and increased CNS viral burden and neuronal injury. This article speaks directly to the hypothesis that asyn is an antimicrobial peptide.
Bantle CM, Phillips AT, Smeyne RJ, Rocha SM, Olson KE, Tjalkens RB. Infection with mosquito-borne alphavirus induces selective loss of dopaminergic neurons, neuroinflammation and widespread protein aggregation. NPJ Parkinsons Dis. 2019;5:20.
Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352(6286):712–6.
Tremblay ME, Cookson MR, Civiero L. Glial phagocytic clearance in Parkinson’s disease. Mol Neurodegener. 2019;14(1):16.
Golde TE, DeKosky ST, Galasko D. Alzheimer’s disease: the right drug, the right time. Science. 2018;362(6420):1250–1.
Jankovic J, Goodman I, Safirstein B, Marmon TK, Schenk DB, Koller M, et al. Safety and tolerability of multiple ascending doses of PRX002/RG7935, an anti-alpha-synuclein monoclonal antibody, in patients with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2018;75(10):1206–14.
Brys M, Fanning L, Hung S, Ellenbogen A, Penner N, Yang M, et al. Randomized phase I clinical trial of anti-alpha-synuclein antibody BIIB054. Mov Disord. 2019;34(8):1154–63.
Chambers TJ, Diamond MS. Pathogenesis of flavivirus encephalitis. Adv Virus Res. 2003;60:273–342.
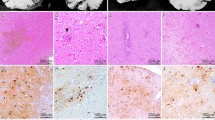
•• Vasek MJ, Garber C, Dorsey D, Durrant DM, Bollman B, Soung A, et al. A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature. 2016;534(7608):538–43 This murine study found impaired spatial learning, attributable to persistent phagocytic microglia that drove complement-mediated synaptic loss in the hippocampus following infection with an attenuated WNV strain. An accompanying examination of human postmortem brain tissue showed reduced CA3 presynaptic terminals in the hippocampus and entorhinal cortex associated with WNV neuroinvasive disease, compared to age-matched controls.
Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74(4):691–705.
Wu T, Dejanovic B, Gandham VD, Gogineni A, Edmonds R, Schauer S, et al. Complement C3 is activated in human AD brain and is required for neurodegeneration in mouse models of amyloidosis and tauopathy. Cell Rep. 2019;28(8):2111–23 e6.
Lian H, Yang L, Cole A, Sun L, Chiang AC, Fowler SW, et al. NFκB-activated astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer’s disease. Neuron. 2015;85(1):101–15.
Canchi S, Raao B, Masliah D, Rosenthal SB, Sasik R, Fisch KM, et al. Integrating gene and protein expression reveals perturbed functional networks in Alzheimer’s disease. Cell Rep. 2019;28(4):1103–16 e4.
Conde JN, Silva EM, Barbosa AS, Mohana-Borges R. The complement system in Flavivirus infections. Front Microbiol. 2017;8:213.
Mehlhop E, Whitby K, Oliphant T, Marri A, Engle M, Diamond MS, et al. Complement activation is required for induction of a protective antibody response against West Nile virus infection. J Virol. 2005;79(12):7466–77.
Seitz S, Clarke P, Tyler KL. Pharmacologic depletion of microglia increases viral load in the brain and enhances mortality in murine models of flavivirus-induced encephalitis. J Virol. 2018;92(16).
Kim IJ, Beck HN, Lein PJ, Higgins D. Interferon gamma induces retrograde dendritic retraction and inhibits synapse formation. J Neurosci. 2002;22(11):4530–9.
Li L, Walker TL, Zhang Y, Mackay EW, Bartlett PF. Endogenous interferon gamma directly regulates neural precursors in the non-inflammatory brain. J Neurosci. 2010;30(27):9038–50.
• Garber C, Soung A, Vollmer LL, Kanmogne M, Last A, Brown J, et al. Tcells promotemicroglia-mediated synaptic elimination and cognitive dysfunction during recovery from neuropathogenic flaviviruses. Nat Neurosci. 2019;22(8):1276–88 In a murine model of West Nile neuroinvasive disease (WNND)-induced cognitive dysfunction, the authors demonstrate that T cell-derived IFN-γ signaling in microglia gives rise to spatial-learning defects and presynaptic termini elimination with lack of repair.
Nott A, Holtman IR, Coufal NG, CM SJ, Yu M, Hu R, et al. Brain cell type-specific enhancer-promoter interactome maps and disease risk association. Science. 2019.
Labzin LI, Heneka MT, Latz E. Innate immunity and neurodegeneration. Annu Rev Med. 2018;69:437–49.
Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med. 2017;23(9):1018–27.
Golde TE. Harnessing immunoproteostasis to treat neurodegenerative disorders. Neuron. 2019;101(6):1003–15.
Chakrabarty P, Ceballos-Diaz C, Beccard A, Janus C, Dickson D, Golde TE, et al. IFN-gamma promotes complement expression and attenuates amyloid plaque deposition in amyloid beta precursor protein transgenic mice. J Immunol. 2010;184(9):5333–43.
Chakrabarty P, Herring A, Ceballos-Diaz C, Das P, Golde TE. Hippocampal expression of murine TNFα results in attenuation of amyloid deposition in vivo. Mol Neurodegener. 2011;6:16.
Chakrabarty P, Jansen-West K, Beccard A, Ceballos-Diaz C, Levites Y, Verbeeck C, et al. Massive gliosis induced by interleukin-6 suppresses Aβ deposition in vivo: evidence against inflammation as a driving force for amyloid deposition. FASEB J. 2010;24(2):548–59.
Chakrabarty P, Li A, Ceballos-Diaz C, Eddy JA, Funk CC, Moore B, et al. IL-10 alters immunoproteostasis in APP mice, increasing plaque burden and worsening cognitive behavior. Neuron. 2015;85(3):519–33.
Chakrabarty P, Tianbai L, Herring A, Ceballos-Diaz C, Das P, Golde TE. Hippocampal expression of murine IL-4 results in exacerbation of amyloid deposition. Mol Neurodegener. 2012;7:36.
Hansen DV, Hanson JE, Sheng M. Microglia in Alzheimer’s disease. J Cell Biol. 2018;217(2):459–72.
• Garber C, Vasek MJ, Vollmer LL, Sun T, Jiang X, Klein RS. Astrocytes decrease adult neurogenesis during virus-induced memory dysfunction via IL-1. Nat Immunol. 2018;19(2):151–61 In a murine model of WNND-induced cognitive dysfunction, the authors demonstrate that there are fewer neuroblasts and increased astrogenesis without recovery of hippocampal neurogenesis at 30 days, mediated by IL-1 secreted predominantly by pro-inflammatory astrocytes.
Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci U S A. 2010;107(42):17872–9.
Sulzer D, Alcalay RN, Garretti F, Cote L, Kanter E, Agin-Liebes J, et al. T cells from patients with Parkinson’s disease recognize alpha-synuclein peptides. Nature. 2017;546(7660):656–61.
Bodea LG, Wang Y, Linnartz-Gerlach B, Kopatz J, Sinkkonen L, Musgrove R, et al. Neurodegeneration by activation of the microglial complement-phagosome pathway. J Neurosci. 2014;34(25):8546–56.
Choi YR, Kang SJ, Kim JM, Lee SJ, Jou I, Joe EH, et al. FcγRIIB mediates the inhibitory effect of aggregated α-synuclein on microglial phagocytosis. Neurobiol Dis. 2015;83:90–9.
Lill CM, Rengmark A, Pihlstrøm L, Fogh I, Shatunov A, Sleiman PM, et al. The role of TREM2 R47H as a risk factor for Alzheimer’s disease, frontotemporal lobar degeneration, amyotrophic lateral sclerosis, and Parkinson’s disease. Alzheimers Dement. 2015;11(12):1407–16.
Rayaprolu S, Mullen B, Baker M, Lynch T, Finger E, Seeley WW, et al. TREM2 in neurodegeneration: evidence for association of the p.R47H variant with frontotemporal dementia and Parkinson’s disease. Mol Neurodegener. 2013;8:19.
Colonna M, Wang Y. TREM2 variants: new keys to decipher Alzheimer disease pathogenesis. Nat Rev Neurosci. 2016;17(4):201–7.
Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, el Fatimy R, et al. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity. 2017;47(3):566–81 e9.
Schäfer A, Brooke CB, Whitmore AC, Johnston RE. The role of the blood-brain barrier during Venezuelan equine encephalitis virus infection. J Virol. 2011;85(20):10682–90.
Obermeier B, Verma A, Ransohoff RM. The blood-brain barrier. Handb Clin Neurol. 2016;133:39–59.
Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14(3):133–50.
Sweeney MD, Kisler K, Montagne A, Toga AW, Zlokovic BV. The role of brain vasculature in neurodegenerative disorders. Nat Neurosci. 2018;21(10):1318–31.
Seo JH, Guo S, Lok J, Navaratna D, Whalen MJ, Kim KW, et al. Neurovascular matrix metalloproteinases and the blood-brain barrier. Curr Pharm Des. 2012;18(25):3645–8.
Shin Y, Choi SH, Kim E, Bylykbashi E, Kim JA, Chung S, et al. Blood-brain barrier dysfunction in a 3D in vitro model of Alzheimer’s disease. Adv Sci (Weinh). 2019;6(20):1900962.
Luo H, Wang T. Recent advances in understanding West Nile virus host immunity and viral pathogenesis. F1000Res. 2018;7:338.
Daniels BP, Holman DW, Cruz-Orengo L, Jujjavarapu H, Durrant DM, Klein RS. Viral pathogen-associated molecular patterns regulate blood-brain barrier integrity via competing innate cytokine signals. MBio. 2014;5(5):e01476–14.
Sonar SA, Shaikh S, Joshi N, Atre AN, Lal G. IFN-gamma promotes transendothelial migration of CD4(+) T cells across the blood-brain barrier. Immunol Cell Biol. 2017;95(9):843–53.
Bonney S, Seitz S, Ryan CA, Jones KL, Clarke P, Tyler KL, et al. Gamma interferon alters junctional integrity via Rho kinase, resulting in blood-brain barrier leakage in experimental viral encephalitis. MBio. 2019;10(4).
Sitati EM, Diamond MS. CD4+ T-cell responses are required for clearance of West Nile virus from the central nervous system. J Virol. 2006;80(24):12060–9.
Wang T, Welte T. Role of natural killer and Gamma-delta T cells in West Nile virus infection. Viruses. 2013;5(9):2298–310.
Frijns CJ, Kappelle LJ. Inflammatory cell adhesion molecules in ischemic cerebrovascular disease. Stroke. 2002;33(8):2115–22.
Wang F, Zou Z, Gong Y, Yuan D, Chen X, Sun T. Regulation of human brain microvascular endothelial cell adhesion and barrier functions by memantine. J Mol Neurosci. 2017;62(1):123–9.
Dudvarski Stankovic N, Teodorczyk M, Ploen R, Zipp F, Schmidt MHH. Microglia-blood vessel interactions: a double-edged sword in brain pathologies. Acta Neuropathol. 2016;131(3):347–63.
Elahy M, Jackaman C, Mamo JC, Lam V, Dhaliwal SS, Giles C, et al. Blood-brain barrier dysfunction developed during normal aging is associated with inflammation and loss of tight junctions but not with leukocyte recruitment. Immun Ageing. 2015;12:2.
Biancardi VC, Son SJ, Ahmadi S, Filosa JA, Stern JE. Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood-brain barrier. Hypertension. 2014;63(3):572–9.
Prasad S, Sajja RK, Naik P, Cucullo L. Diabetes mellitus and blood-brain barrier dysfunction: an overview. Aust J Pharm. 2014;2(2):125.
Badawi A, Velummailum R, Ryoo SG, Senthinathan A, Yaghoubi S, Vasileva D, et al. Prevalence of chronic comorbidities in dengue fever and West Nile virus: a systematic review and meta-analysis. PLoS One. 2018;13(7):e0200200.
Hart J Jr, Tillman G, Kraut MA, Chiang HS, Strain JF, Li Y, et al. NIAID Collaborative Antiviral Study Group West Nile Virus 210 Protocol Team. West Nile virus neuroinvasive disease: neurological manifestations and prospective longitudinal outcomes. BMC Infect Dis. 2014;14:248.
Jean CM, Honarmand S, Louie JK, Glaser CA. Risk factors for West Nile virus neuroinvasive disease, California, 2005. Emerg Infect Dis. 2007;13(12):1918–20.
Ronca SE, Dineley KT, Paessler S. Neurological sequelae resulting from encephalitic alphavirus infection. Front Microbiol. 2016;7:959.
Lebov JF, Brown LM, MacDonald P, Robertson K, Bowman NM, Hooper SR, et al. Review: evidence of neurological sequelae in children with acquired Zika virus infection. Pediatr Neurol. 2018;85:16–20.
Liddelow SA, Barres BA. Reactive astrocytes: production, function, and therapeutic potential. Immunity. 2017;46(6):957–67.
Clarke LE, Liddelow SA, Chakraborty C, Münch AE, Heiman M, Barres BA. Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci U S A. 2018;115(8):E1896–905.
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–7.
Itzhaki RF, Lin WR, Shang D, Wilcock GK, Faragher B, Jamieson GA. Herpes simplex virus type 1 in brain and risk of Alzheimer’s disease. Lancet. 1997;349(9047):241–4.
Jamieson GA, Maitland NJ, Wilcock GK, Craske J, Itzhaki RF. Latent herpes simplex virus type 1 in normal and Alzheimer’s disease brains. J Med Virol. 1991;33(4):224–7.
Lövheim H, Gilthorpe J, Adolfsson R, Nilsson LG, Elgh F. Reactivated herpes simplex infection increases the risk of Alzheimer’s disease. Alzheimers Dement. 2015;11(6):593–9.
Lövheim H, Norman T, Weidung B, Olsson J, Josefsson M, Adolfsson R, et al. Herpes simplex virus, APOEvarepsilon4, and cognitive decline in old age: results from the Betula cohort study. J Alzheimers Dis. 2019;67(1):211–20.
Miklossy J, Khalili K, Gern L, Ericson RL, Darekar P, Bolle L, et al. Borrelia burgdorferi persists in the brain in chronic lyme neuroborreliosis and may be associated with Alzheimer disease. J Alzheimers Dis. 2004;6(6):639–49 discussion 673-81.
Stanley LC, Mrak RE, Woody RC, Perrot LJ, Zhang S, Marshak DR, et al. Glial cytokines as neuropathogenic factors in HIV infection: pathogenic similarities to Alzheimer’s disease. J Neuropathol Exp Neurol. 1994;53(3):231–8.
Esiri MM, Biddolph SC, Morris CS. Prevalence of Alzheimer plaques in AIDS. J Neurol Neurosurg Psychiatry. 1998;65(1):29–33.
Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS. 2005;19(4):407–11.
Balin BJ, Gérard HC, Arking EJ, Appelt DM, Branigan PJ, Abrams JT, et al. Identification and localization of Chlamydia pneumoniae in the Alzheimer’s brain. Med Microbiol Immunol. 1998;187(1):23–42.
Dominy SS, Lynch C, Ermini F, Benedyk M, Marczyk A, Konradi A, et al. Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. 2019;5(1):eaau3333.
Readhead B, Haure-Mirande JV, Funk CC, Richards MA, Shannon P, Haroutunian V, et al. Multiscale analysis of independent Alzheimer’s cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron. 2018;99(1):64–82 e7.
Vilensky JA, Gilman S, McCall S. Does the historical literature on encephalitis lethargica support a simple (direct) relationship with postencephalitic Parkinsonism? Mov Disord. 2010;25(9):1124–30.
Takahashi M, Yamada T. Viral etiology for Parkinson’s disease--a possible role of influenza a virus infection. Jpn J Infect Dis. 1999;52(3):89–98.
Adams B, Nunes JM, Page MJ, Roberts T, Carr J, Nell TA, et al. Parkinson’s disease: a systemic inflammatory disease accompanied by bacterial inflammagens. Front Aging Neurosci. 2019;11:210.
Shen X, Yang H, Wu Y, Zhang D, Jiang H. Meta-analysis: association of Helicobacter pylori infection with Parkinson’s diseases. Helicobacter. 2017;22(5).
Wijarnpreecha K, Chesdachai S, Jaruvongvanich V, Ungprasert P. Hepatitis C virus infection and risk of Parkinson’s disease: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30(1):9–13.
Matheoud D, Cannon T, Voisin A, Penttinen AM, Ramet L, Fahmy AM, et al. Intestinal infection triggers Parkinson’s disease-like symptoms in Pink1(-/-) mice. Nature. 2019;571(7766):565–9.
Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9(2):106–18.
Burgos JS, Ramirez C, Sastre I, Valdivieso F. Effect of apolipoprotein E on the cerebral load of latent herpes simplex virus type 1 DNA. J Virol. 2006;80(11):5383–7.
Hoshino Y, Pesnicak L, Cohen JI, Straus SE. Rates of reactivation of latent herpes simplex virus from mouse trigeminal ganglia ex vivo correlate directly with viral load and inversely with number of infiltrating CD8+ T cells. J Virol. 2007;81(15):8157–64.
Martins AS, Carvalho FA, Faustino AF, Martins IC, Santos NC. West Nile virus capsid protein interacts with biologically relevant host lipid systems. Front Cell Infect Microbiol. 2019;9:–8.
Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K. Gut microbes and the brain: paradigm shift in neuroscience. J Neurosci. 2014;34(46):15490–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Emerging Tropical Diseases
Rights and permissions
About this article
Cite this article
Vittor, A.Y., Long, M., Chakrabarty, P. et al. West Nile Virus-Induced Neurologic Sequelae—Relationship to Neurodegenerative Cascades and Dementias. Curr Trop Med Rep 7, 25–36 (2020). https://doi.org/10.1007/s40475-020-00200-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40475-020-00200-7