Abstract
Despite the significant reduction in global leprosy prevalence in the advent of multidrug therapy, epidemiological evidence shows that new case detection rate in endemic countries is still substantial. In the past three decades, there has been much research effort into finding more sensitive and specific laboratory techniques for the early detection of leprosy infection to prevent the cutaneous eruptions and severe disabling neurological sequelae. Correct classification of leprosy subtypes for the purpose of instituting the appropriate drug regimen can only be achieved by including laboratory investigations with the clinical findings. Unfortunately, this is not always possible in resource restricted endemic regions. In this review, we aim to provide an overview of the traditional laboratory methods that have been in use as well as the newer serological and molecular tools that have shown potential in improving specificity and sensitivity of leprosy diagnosis. How these new diagnostic aids may contribute to the study of reactions in leprosy and of the monitoring of treatment efficacy, disease relapse, transmission, and risk of disease manifestation in household contacts will also be outlined in this article.
Similar content being viewed by others
Introduction
With the introduction of multidrug therapy (MDT), elimination of leprosy, which is defined as <1 case per 10,000 population, was achieved at global level by the year 2000. However, the new case detection rate and disease burden associated with leprosy are still significant in endemic countries [1]. This continual transmission of leprosy is due in part to delayed diagnosis and management of contacts. Delayed diagnosis and slow recognition of reactional episodes in turn leads to more severe nerve damage and scarring [2, 3]. Diagnosing leprosy is straightforward when classic features such as erythematous or hypopigmented skin lesions, anesthesia, nerve thickening, and neuropathy are present. However, in cases where skin lesions are subtle or inconclusive, laboratory investigations are warranted for confirmation of the disease. In 1966, Ridley and Jopling devised the five-group classification system of leprosy based on the correlation of histology with clinical and immunological features and bacterial index (BI) [4]. This system delineates the tuberculoid and lepromatous poles of leprosy and their borderline forms where the categorization reflects the degree and type of the individual’s immune response to the infection. Since bacterial smears are not always available, the WHO recommended in 1997 a simple operational classification system to divide leprosy patients into paucibacillary (PB) and multibacillary (MB) groups based on the number of skin lesions, with a further single-lesion only PB category. The following sections will describe the histology of leprosy and the laboratory tests currently available or under investigation for leprosy diagnosis and research.
Histology
A study of 1265 leprosy patient reports 58.1 % concordance between histological findings and subtyping based only on clinical features according to the WHO operational system [5]. Another study showed that only 74.7 % of suspected cases of leprosy had histology corroborating with the clinical subtyping [6]. In both studies, the best correlated form is the lepromatous subtype. They serve to highlight the importance of histological examination in placing patients into the correct categories so that they receive the appropriate MDT regimen and thus reducing deformities and disease transmission.
We will briefly examine the histological features of the different subtypes of leprosy based on the Ridley and Jopling classification:
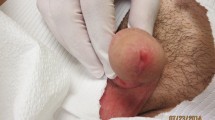
Tuberculoid leprosy (TT) is characterized by multiple, well-formed, noncaseating granulomas in the dermis rimmed by lymphocytes (Fig. 1). These granulomas are composed of epithelioid histiocytes which may fuse to form multinucleate Langhans giant cells. The inflammatory process extends to the epidermis. Nerve bundles are not well seen within the granulomas and may require S100 immunostain for identification. Acid-fast bacilli (AFB) are absent. Borderline turberculoid leprosy (BT) shares the granulomatous appearance with the TT group, but a grenz zone is present, even if it is very narrow. The granulomas are cuffed by more lymphocytes than those seen in the BB type described below, but less so than those in TT. Swollen nerve bundles may be seen within the granulomas. Bacillary index (BI) is 0 to 2+ in the granuloma or 1 to 3+ in the involved nerve bundles, on the logarithmic scale of 1+ to 6+ with 1+ being at least 1 bacillus in every 100 fields at ×100 oil immersion magnification, and 6+ being at least 1000 bacilli in every field. In the borderline (BB) group, the epithelioid histiocytes are diffusely disposed and are not as large as those in TT type. Langhans giant cells are absent, and lymphocytes may be present diffusely. It is not uncommon to find plasma cells within the inflammatory infiltrate. Nerve bundles show moderate Schwann cell proliferation, and the BI is often 3 to 4 +. In the borderline lepromatous (BL) group, there are aggregates of lymphocytes and macrophages with granular to foamy cytoplasm. The number of lymphocytes is variable and may be present around small dermal nerves. The BI is usually 5+. Lepromatous leprosy (LL) consists of sheets of foamy histiocytes (Virchow cells) containing numerous AFB (Figs. 2 and 3), which sometimes take the form of large clumps called globi. These globi are present within the cytoplasm of the enlarged, foamy histiocytes. Lymphocytes are scanty. Nerve bundles may show structural damage but lack cellular infiltrate. This subtype corresponds to the high BI, typically 5+. There is often a grenz zone separating the inflammatory infiltrate and the epidermis.
The indeterminate group refers to the early cases of leprosy when the immunological response is beginning to take place. There is a sparsely cellular infiltrate of perivascular and periadnexal lymphocytes and histiocytes. Bacilli are usually scanty or absent. The histioid variant is a multibacilliary form consisting of spindle cells filled with bacilli that line up along the long axis of the cells. Special stains to highlight the bacteria are required to distinguish this from other differential diagnoses such as tuberculosis, atypical mycobacterial infections, noninfectious sarcoidal granulomas, Crohn’s disease, and xanthomas [7, 8].
In addition to providing a morphological diagnosis, skin histology allows bacterial quantification by Ziehl-Neelsen (ZN), Fite Faraco stains, and immunohistochemistry. Previous studies have demonstrated the use of immunohistochemistry in detecting Mycobacterium leprae antigens in the frozen section skin tissue [9, 10]. Localization of the stain is within macrophages and is more commonly positive in the MB subtypes as expected. In situ hybridization in skin smears has also been shown to enhance the diagnosis of leprosy when compared with ZN staining in skin smears [11].
Pure Neural Leprosy
Pure neural leprosy (PNL) is difficult to diagnose due to the lack of skin lesions. Arrival at the correct diagnosis requires correlation with nerve biopsies and demonstration of AFB, as well as molecular detection if necessary. However, some histological signs can still be useful for the diagnosis of PNL even in the absence of AFB. These include epithelioid granulomas, mononuclear cell infiltrate, perineural/subperineural edema, fibrosis, and a decrease in myelinated fibers [12]. Immunohistochemistry using antibodies against lipoarabinomanan and phenolic glycolipid 1 (PGL-1), which are structural and highly antigenic mycobacterial wall components involved in the regulation of cell mediated response to M. leprae, has been shown to be useful when AFB cannot be demonstrated in the nerve sample [13].
Reactions
Three important reactions can take place during the course of leprosy, often after commencement of MDT. They are more commonly seen in the lepromatous and borderline groups of patients. Type I reversal reaction is the most important cause of nerve damage and permanent disability, and it is characterized by abrupt inflammatory skin and nerve changes associated with spontaneous nerve pain and nerve function damage. It is predominantly a clinical diagnosis but histological features such as granulomatous destruction of the nerves, and dermal edema can help support the diagnosis [14–16]. Early recognition of these acute reactional episodes is imperative for prompt management and prevention of permanent disabilities. Type 2 erythema nodosum leprosum reaction is an immune complex-mediated and cell-mediated immune phenomenon characterized by rapid onset of painful erythematous subcutaneous nodules which may ulcerate. It can be accompanied by fever and malaise, as well as systemic involvement such as iritis, arthritis, lymphadenitis, and neuritis. Histology shows dermal neutrophils with possible neutrophilic lobular panniculitis and vasculitis. Bacilli-laden foamy macrophages are seen [14, 17]. The third reaction pattern, the Lucio phenomenon, is a necrotizing vasculitis presenting with abrupt onset of painful macules to necrotic ulcer. Infiltration of endothelial cells by M. leprae bacilli-laden macrophages can be seen on histology [18].
Slit Skin Smears and Intradermal Skin Tests
In slit skin smears, fluid obtained from cutaneous lesions is smeared and dried on a glass slide and then stained with Fite stain. The number of bacteria is counted under light microscopy at high magnification with oil immersion to provide a bacterial index using a logarithmic scale [4]. Since BI is high in MB patients and often negative in PB patients, the use of slit skin smears is limited to classification for treatment purposes and cannot be extended as a standalone diagnostic test for leprosy [19].
Along the same line, the lepromin test first described by Mitsuda in 1919 [20] detects only the cellular immune response to intradermal injection of heat killed M. leprae bacilli, and hence it is only effective in the diagnosis of PB leprosy but not MB. Its role is therefore also limited to classification of leprosy. Recently, there has been interest in the search for candidate synthetic peptides [21] that may provide an alternative source of antigens for the lepromin test. MLSA-LAM (M. leprae soluble antigen devoid of mycobacterial lipoglycans, primarily lipoarabinomannan) and MLCwA (M. leprae cell wall antigens) are two such candidates [22], and they consist of the immunologically active proteins from the soluble/cytosol and insoluble cell wall of M. leprae. Other intradermal tests such as the histamine and pilocarpine tests assess the integrity of neural function [23] in leprosy and are not diagnostic screening tools for the general population. In the histamine test, the lack of a red dermal flare after injection of histamine signifies nerve damage, while the lack of sweating is associated with nerve damage in the pilocarpine test.
Serology
In the 1980s, numerous research studies looked into possible M. leprae-specific antigens with the hope of developing serodiagnostic reagents for leprosy confirmation when clinical features are inconclusive, and other tests such as slit skin smears and histology are unavailable. The most widely investigated antigen is phenolic glycolipid I (PGL-I) [24, 25], which was demonstrated to be serologically active by enzyme-linked immunosorbent assay (ELISA) [26]. Further studies led to the development of ELISA assays against semi-synthetic derivatives of PGL-I comprising the natural di- or trisaccharide of phenolic glycolipid and a bovine serum albumin (BSA) protein carrier connected by a octyl and phenyl linker, respectively [27, 28]. Since the majority of PB patients are seronegative and majority of MB patients are seropositive, testing for PGL-I antibody is useful for disease classification for treatment purposes. However, it is unsuitable as a screening diagnostic test due to its low sensitivity for PB leprosy [29]. Since ELISA-based assays are inconvenient in field settings, a simpler method called the gel particle agglutination method (MLPA) was devised that studies the agglutination of gelatin particles sensitized with the trisaccharide-based antigen, in the presence of patient’s sera [30]. A simple card test kit for the detection of serological response to the recombinant M. leprae-specific 35 kDa protein was also found to be a good simple serodiagnostic aid [31–33]. It uses a cardboard folder containing a nitrocellulose strip with the 35 kDa antigen, on which patient’s sera can be applied and allowed to diffuse down, producing a red line if the result is positive. Following the discontinuation of MLPA and the 35 kDa card test kit, investigators looked further for alternative methods to overcome the problem of the slow turnaround time of ELISA, which typically takes 1 day to run [34]. The dipstick serum-based [35] and whole blood-based [36] assays which have a turnaround time of 3 h [34], and the lateral flow test [34, 37, 38] which only takes 10 min to run, are such alternatives. A recent study demonstrated human serum albumin (HSA) [39] to be an effective substitute to BSA as the protein carrier for semisynthetic PGL-I.
With the knowledge of the complete M. leprae genome sequence, researchers began searching for species-specific protein antigens that may be useful in the serodiagnosis of leprosy [40]. Antibody to LID-1, a recombinant fusion protein combining the antigens ML0405 and ML2331, was found to be a robust serological marker for MB leprosy [41]. Antibody response against LID-1 coupled with the natural disaccharide octyl (NDO-LID) was shown to be more effective in identifying subclinical infection when compared with the serological response against NDO-HSA alone [42]. These recombinant protein-based serological test kits have also been tested with cell phone-based Smart Reader technology [43, 44] to provide more consistent and quantifiable results. This android-based smartphone rapid test reader platform mechanically attached to the existing camera unit, collects test images, and objectively quantifies signal intensities of the control and test lines in each NDO-LID test.
Some studies suggested that seropositive contacts are at higher risk for developing leprosy [30, 34, 45], and therefore, serological tests may be of value in this aspect. However, this requires further evaluation. While species-specific antibody response may have a role in assessing treatment efficacy and disease relapse [46], its utility in predicting reactions in leprosy is still a subject for investigation [34].
PCR
Since M. leprae cannot be successfully grown in culture, the use of polymerase chain reaction (PCR) to amplify M. leprae DNA in clinical samples has been extensively investigated. An earlier study amplifying the 530 bp gene fragment encoding the 36 kDa species-specific proline-rich antigen in purified M. leprae sample showed that it has a detection limit approximating 1 to 10 bacilli [47]. PCR amplification of this gene target in neutral formalin-fixed skin biopsies yielded positive result in 92 % of untreated acid-fast bacilli (AFB)-positive patients and in 61 % of untreated AFB-negative patients [48]. This PCR method was also shown to have higher sensitivity than BI from slit skin smears and serological testing for anti-PGL-I antibody, even in the PB patients [49]. Other studies which amplified the 360 bp gene fragment encoding the M. leprae 18 kDa protein [50] and the M. leprae-specific repetitive sequence (RLEP) [51] also yielded good specificity. In difficult diagnoses of PNL, PCR adds sensitivity to conventional histological methods [52] and fine needle aspiration [53]. Banerjee et al. found that multiplex PCR was a sensitive method of detecting M.leprae and has potential in assessing contact cases for risk of disease development [54]. However, this requires longer follow-up of contacts for further evaluation. A review article by Martinez et al. collated the results of all the different DNA-based PCR assays on skin biopsies and illustrated up to 100 % specificity for leprosy, and 34 to 80 % and more than 90 % sensitivity for PB and MB patients, respectively [55•].
Real-time PCR technology has the added ability to rapidly detect and quantify M. leprae DNA which is not detectable by conventional histological staining and can be used to differentiate MB from PB leprosy [56]. In particular, molecular enumeration of M. leprae bacterial load with real-time PCR of the RLEP gene fragment [57] was shown to have good sensitivity for detection of early M. leprae infection before major clinical manifestations [58]. Furthermore, simultaneous quantitative reverse transcriptase PCR of 16S rRNA and RLEP appears to be useful in monitoring bacterial viability [59–61] and hence, drug efficacy. Another group found that real-time PCR coupled with high-resolution melt analysis may facilitate the study of drug resistance and strain typing [62]. In relation to contact risk assessment, Reis et al. demonstrated that contacts with positive quantitative PCR of ML 0024 in blood signified an increased risk of disease development during a 7-year follow-up [63]. Used in conjunction with other prognostic markers, this test may be beneficial in the management of contacts, but this will require further evaluation. A recent interesting study suggested that an increase in mRNA expression of vitamin D receptor is possibly related to type 2 reactions in leprosy [64].
With the recent advances in genomic studies, candidate genes that appear to be associated with susceptibility to leprosy development have been identified. These include TLR1 (toll-like receptor 1), TLR2 (toll-like receptor 2), HLA-DR, NRAMP1 (encoding natural resistance-associated macrophage protein 1), PARK2 (encoding parkin), lymphotoxin alpha, and NOD2 (nucleotide-binding oligomerization domain-containing protein 2) [65•]. These may have a future role in leprosy risk assessment in endemic regions and in research.
Although PCR techniques seem to be more specific and sensitive, they are also more expensive than the traditional laboratory methods of slit skin smears and histology. Therefore, they are not suited to field diagnosis and should be reserved only for difficult diagnostic cases such as subtle PB cases, indeterminate leprosy, and pure neural leprosy, especially in resource-limited regions. One cannot solely rely on PCR as there is still a significant false-negative rate.
Conclusion
The diagnosis of leprosy is still primarily based on clinical features, and while all of the laboratory tests discussed can help clinch the diagnosis and subtype the disease, none are considered enough as standalone diagnostic tests. There is still room for improvement in the development of laboratory techniques for the early detection of leprosy to prevent severe disfigurement and neurological damage. Better identification of high-risk contacts may have a role in the management of contacts once the efficacy of chemoprophylaxis [66] and immunoprophylaxis with BCG vaccination [67] in leprosy contacts is thoroughly evaluated. The expansion of new molecular tools can give additional insight into the immunology and pathogenesis of leprosy, as well as increase our understanding of drug resistance, which is important when there is no novel drug in development for leprosy at the present moment. The cumulative knowledge on the M. leprae genome also paves the road for vaccine development in the future. With the reduction in the global prevalence of leprosy, clinicians are becoming less familiar with the disease and are less able to diagnose leprosy promptly and institute treatment early. However, due to common international migration nowadays, physicians in developed countries need to maintain a high index of suspicion when faced with patients from endemics regions who present with concurrent dermatological and neurological symptoms, and they should be aware of the laboratory tests available.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Global leprosy update, 2013; reducing disease burden. Wkly Epidemiol Rec. 2014 Sep 5;89(36):389-400
Rodrigues LC, Lockwood DNJ. Leprosy now: epidemiology, progress, challenges, and research gaps. Lancet Infect Dis. 2011;11(6):464–70.
Yadav N, Kar S, Madke B, Dashatwar D, Singh N, Prasad K, et al. Leprosy elimination: a myth busted. J Neurosci Rural Pract. 2014;5 Suppl 1:S28–32.
Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966;34(3):255–73.
Santos VS, de Mendonça Neto PT, Falcão Raposo OF, Fakhouri R, Reis FP, Feitosa VL. Evaluation of agreement between clinical and histopathological data for classifying leprosy. Int J Infect Dis. 2013;17(3):e189–92.
Shivaswamy KN, Shyamprasad AL, Sumathy TK, Ranganathan C, Agarwal V. Clinico histopathological correlation in leprosy. Dermato Online J. 2012;18(9):2.
Da Costa DA, Enokihara MM, Nonogaki S, Maeda SM, Porro AM, Tomimori J. Wade histoid leprosy: histological and immunohistochemical analysis. Lepr Rev. 2013;84(3):176–85.
Nair SP, Nanda KG. A clinical and histopathological study of histoid leprosy. Int J Dermatol. 2013;52(5):580–6.
Khanolkar SR, Mackenzie CD, Lucas SB, Hussen A, Girdhar BK, Katoch K, et al. Identification of Mycobacterium leprae antigens in tissues of leprosy patients using monoclonal antibodies. Int J Lepr Other Mycobact Dis. 1989;57(3):652–8.
Verhagen C, Faber W, Klatser P, Buffing A, Naafs B, Das P. Immunohistological analysis of in situ expression of mycobacterial antigens in skin lesions of leprosy patients across the histopathological spectrum. Association of Mycobacterial lipoarabinomannan (LAM) and Mycobacterium leprae phenolic glycolipid-I (PGL-I) with leprosy reactions. Am J Pathol. 1999;154(6):1793–804.
Kamal R, Natrajan M, Katoch K, Parvez M, Nag VK, Dayal R. Evaluation of the diagnostic value of immunocytochemistry and in situ hybridization in the pediatric leprosy. Indian J Lepr. 2013;85(3):109–14.
Antunes SL, Chimelli L, Jardim MR, Vital RT, Nery JA, Corte-Real S, et al. Histopathological examination of nerve samples from pure neural leprosy patients: obtaining maximum information to improve diagnostic efficiency. Mem Inst Oswaldo Cruz. 2012;107(2):246–53.
Ferreira Medeiros M, Jardim MR, Vital RT, Nery JA, Sales AM, de Moraes MO, et al. An attempt to improve pure neural leprosy diagnosis using immunohistochemistry tests in peripheral nerve biopsy specimens. Appl Immunohistochem Mol Morphol. 2014;22(3):222–30.
Kamath S, Vaccaro SA, Rea TH, Ochoa MT. Recognizing and managing the immunologic reactions in leprosy. J Am Acad Dermatol. 2014;71(4):795–803.
Nery JA, Bernardes Filho F, Quintanilha J, Machado AM, Oliveira Sde S, Sales AM. Understanding the type 1 reactional state for early diagnosis and treatment: a way to avoid disability in leprosy. An Bras Dermatol. 2013;88(5):787–92.
Lockwood DN, Lucas SB, Desikan KV, Ebenezer G, Suneetha S, Nicholls P. The histological diagnosis of leprosy type 1 reactions: identification of key variables and an analysis of the process of histological diagnosis. J Clin Pathol. 2008;61(5):595–600.
Kahawita IP, Lockwood DN. Towards understanding the pathology of erythema nodosum leprosum. Trans R Soc Trop Med Hyg. 2008;102(4):329–37.
Magaña M, Fernández-Díez J, Magaña ML. Lucio’s phenomenon is a necrotizing panvasculitis: mostly a medium-sized granulomatous arteritis. Am J Dermatopathol. 2008;30(6):555–60.
Lastória JC, Abreu MA. Leprosy: a review of laboratory and therapeutic aspects - part 2. An Bras Dermatol. 2014;89(3):389–403.
Wade HW. Origin of the lepromin test. Int J Lepr. 1951;19(2):221–4.
Alban SM, de Moura JF, Minozzo JC, Mira MT, Soccol VT. Identification of mimotopes of Mycobacterium leprae as potential diagnostic reagents. BMC Infect Dis. 2013 Jan;13(42).
Rivoire BL, TerLouw S, Groathouse NA, Brennan PJ. The challenge of producing skin test antigens with minimal resources suitable for human application against a neglected tropical disease; leprosy. PLoS Negl Trop Dis. 2014;8(5):e2791.
Pande S, Nagar R, Khopkar U. Intradermal tests in dermatology-II: tests for noninfectious diseases. Indian J Dermatol Venereol Leprol. 2007;73(1):57–9.
Brennan PJ, Barrow WW. Evidence for species-specific lipid antigens in Mycobacterium leprae. Int J Lepr Other Mycobact Dis. 1980;48(4):382–7.
Hunter SW, Brennan PJ. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J Bacteriol. 1981;147(3):728–35.
Brett SJ, Draper P, Payne SN, Rees RJ. Serological activity of a characteristic phenolic glycolipid from Mycobacterium leprae in sera from patients with leprosy and tuberculosis. Clin Exp Immunol. 1983;52(2):271–9.
Cho SN, Yanagihara DL, Hunter SW, Gelber RH, Brennan PJ. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect Immun. 1983;41(3):1077–83.
Fujiwara T, Hunter SW, Cho SN, Aspinall GO, Brennan PJ. Chemical synthesis and serology of disaccharides and trisaccharides of phenol glycolipid antigens from the leprosy bacillus and preparation of a disaccharide protein conjugate for serodiagnosis of leprosy. Infect Immun. 1984;43(1):245–52.
Burgess PJ, Fine PE, Ponnighaus JM, Draper C. Serological tests in leprosy. The sensitivity, specificity and predictive value of ELISA tests based on phenolic glycolipid antigens, and the implications for their use in epidemiological studies. Epidemiol Infect. 1988;101(1):159–71.
Izumi S, Fujiwara T, Ikeda M, Nishimura Y, Sugiyama K, Kawatsu K. Novel gelatin particle agglutination test for serodiagnosis of leprosy in the field. J Clin Microbiol. 1990;28(3):525–9.
Roche PW, Failbus SS, Britton WJ, Cole R. Rapid method for diagnosis of leprosy by measurements of antibodies to the M. leprae 35-kDa protein: comparison with PGL-I antibodies detected by ELISA and “dipstick” methods. Int J Lepr Other Mycobact Dis. 1999;67(3):279–86.
Triccas JA, Roche PW, Winter N, Feng CG, Butlin CR, Britton WJ. A 35-kilodalton protein is a major target of the human immune response to Mycobacterium leprae. Infect Immun. 1996;64(12):5171–7.
Triccas JA, Roche PW, Britton WJ. Specific serological diagnosis of leprosy with a recombinant Mycobacterium leprae protein purified from a rapidly growing mycobacterial host. J Clin Microbiol. 1998;36(8):2363–5.
Oskam L, Slim E, Bührer-Sékula S. Serology: recent developments, strengths, limitations and prospects: a state of the art overview. Lepr Rev. 2003;74(3):196–205.
Bührer SS, Smits HL, Gussenhoven GC, van Ingen CW, Klatser PR. A simple dipstick assay for the detection of antibodies to phenolic glycolipid-I of mycobacterium leprae. Am J Trop Med Hyg. 1998;58(2):133–6.
Bührer-Sekula S, Cunha MG, Ferreira WA, Klatser PR. The use of whole blood in a dipstick assay for detection of antibodies to Mycobacterium leprae: a field evaluation. FEMS Immunol Med Microbiol. 1998;21(3):197–201.
Bührer-Sékula S, Smits HL, Gussenhoven GC, van Leeuwen J, Amador S, Fujiwara T, et al. Simple and fast lateral flow test for classification of leprosy patients and identification of contacts with high risk of developing leprosy. J Clin Microbiol. 2003;41(5):1991–5.
Contin LA, Alves CJ, Fogagnolo L, Nassif PW, Barreto JA, Lauris JR, et al. Use of the ML-Flow test as a tool in classifying and treating leprosy. An Bras Dermatol. 2011;86(1):91–5.
Moura RS, Penna GO, Fujiwara T, Pontes MA, Cruz R, Gonçalves Hde S, et al. Evaluation of a rapid serological test for leprosy classification using human serum albumin as the antigen carrier. J Immunol Methods. 2014;412:35–41.
Groathouse NA, Amin A, Marques MA, Spencer JS, Gelber R, Knudson DL, et al. Use of protein microarrays to define the humoral immune response in leprosy patients and identification of disease-state-specific antigenic profiles. Infect Immun. 2006;74(11):6458–66.
Duthie MS, Ireton GC, Kanaujia GV, Goto W, Liang H, Bhatia A, et al. Selection of antigens and development of prototype tests for point-of-care leprosy diagnosis. Clin Vaccine Immunol. 2008;15(10):1590–7.
da Conceição Oliveira Coelho Fabri A, Carvalho AP, Araujo S, Goulart LR, de Mattos AM, Teixeira HC, et al. Antigen-specific assessment of the immunological status of various groups in a leprosy endemic region. BMC Infect Dis. 2015;15:218.
Paula Vaz Cardoso L, Dias RF, Freitas AA, Hungria EM, Oliveira RM, Collovati M. Development of a quantitative rapid diagnostic test for multibacillary leprosy using smart phone technology. BMC Infect Dis. 2013;13:497.
Duthie MS, Balagon MF, Maghanoy A, Orcullo FM, Cang M, Dias RF, et al. Rapid quantitative serological test for detection of infection with Mycobacterium leprae, the causative agent of leprosy. J Clin Microbiol. 2014;52(2):613–9.
Düppre NC, Camacho LA, Sales AM, Illarramendi X, Nery JA, Sampaio EP, et al. Impact of PGL-I seropositivity on the protective effect of BCG vaccination among leprosy contacts: a cohort study. PLoS Negl Trop Dis. 2012;6(6):e1711.
Duthie MS, Hay MN, Rada EM, Convit J, Ito L, Oyafuso LK, et al. Specific IgG antibody responses may be used to monitor leprosy treatment efficacy and as recurrence prognostic markers. Eur J Clin Microbiol Infect Dis. 2011;30(10):1257–65.
Hartskeerl RA, de Wit MY, Klatser PR. Polymerase chain reaction for the detection of Mycobacterium leprae. J Gen Microbiol. 1989;135(9):2357–64.
de Wit MY, Faber WR, Krieg SR, Douglas JT, Lucas SB, Montreewasuwat N, et al. Application of a polymerase chain reaction for the detection of Mycobacterium leprae in skin tissues. J Clin Microbiol. 1991;29(5):906–10.
Wichitwechkarn J, Karnjan S, Shuntawuttisettee S, Sornprasit C, Kampirapap K, Peerapakorn S. Detection of Mycobacterium leprae infection by PCR. J Clin Microbiol. 1995;33(1):45–9.
Williams DL, Gillis TP, Booth RJ, Looker D, Watson JD. The use of a specific DNA probe and polymerase chain reaction for the detection of Mycobacterium leprae. J Infect Dis. 1990;162(1):193–200.
Santos AR, De Miranda AB, Sarno EN, Suffys PN, Degrave WM. Use of PCR-mediated amplification of Mycobacterium leprae DNA in different types of clinical samples for the diagnosis of leprosy. J Med Microbiol. 1993;39(4):298–304.
Garbino JA, Marques Jr W, Barreto JA, Heise CO, Rodrigues MM, Antunes SL, et al. Primary neural leprosy: systematic review. Arq Neuropsiquiatr. 2013;71(6):397–404.
Reja AH, De A, Biswas S, Chattopadhyay A, Chatterjee G, Bhattacharya B, et al. Use of fine needle aspirate from peripheral nerves of pure-neural leprosy for cytology and PCR to confirm the diagnosis: a pilot study. Indian J Dermatol Venereol Leprol. 2013;79(6):789–94.
Banerjee S, Sarkar K, Gupta S, Mahapatra PS, Gupta S, Guha S, et al. Multiplex PCR technique could be an alternative approach for early detection of leprosy among close contacts—a pilot study from India. BMC Infect Dis. 2010;10:252.
Martinez AN, Talhari C, Moraes MO, Talhari S. PCR-based techniques for leprosy diagnosis: from the laboratory to the clinic. PLoS Negl Trop Dis. 2014;8(4):e2655. A good summary of the different PCR assays with potential use in leprosy diagnosis.
Martinez AN, Britto CF, Nery JA, Sampaio EP, Jardim MR, Sarno EN, et al. Evaluation of real-time and conventional PCR targeting complex 85 genes for detection of Mycobacterium leprae DNA in skin biopsy samples from patients diagnosed with leprosy. J Clin Microbiol. 2006;44(9):3154–9.
Truman RW, Andrews PK, Robbins NY, Adams LB, Krahenbuhl JL, Gillis TP. Enumeration of Mycobacterium leprae using real-time PCR. PLoS Negl Trop Dis. 2008;2(11):e328.
Martinez AN, Ribeiro-Alves M, Sarno EN, Moraes MO. Evaluation of qPCR-based assays for leprosy diagnosis directly in clinical specimens. PLoS. 2011;5(10):e1354.
Martinez AN, Lahiri R, Pittman TL, Scollard D, Truman R, Moraes MO, et al. Molecular determination of Mycobacterium leprae viability by use of real-time PCR. J Clin Microbiol. 2009;47(7):2124–30.
Kurabachew M, Wondimu A, Ryon JJ. Reverse transcription-PCR detection of Mycobacterium leprae in clinical specimens. J Clin Microbiol. 1998;36(5):1352–6.
Phetsuksiri B, Rudeeaneksin J, Supapkul P, Wachapong S, Mahotarn K, Brennan PJ. A simplified reverse transcriptase PCR for rapid detection of Mycobacterium leprae in skin specimens. FEMS Immunol Med Microbiol. 2006;48(3):319–28.
Li W, Matsuoka M, Kai M, Thapa P, Khadge S, Hagge DA, et al. Real-time PCR and high-resolution melt analysis for rapid detection of Mycobacterium leprae drug resistance mutations and strain types. J Clin Microbiol. 2012;50(3):742–53.
Reis EM, Araujo S, Lobato J, Neves AF, Costa AV, Gonçalves MA, et al. Mycobacterium leprae DNA in peripheral blood may indicate a bacilli migration route and high-risk for leprosy onset. Clin Microbiol Infect. 2014;20(5):447–52.
Mandal D, Reja AH, Biswas N, Bhattacharyya P, Patra PK, Bhattacharya B. Vitamin D receptor expression levels determine the severity and complexity of disease progression among leprosy reaction patients. New Microbes New Infect. 2015;6:35–9.
Nath I, Saini C, Valluri VL. Immunology of leprosy and diagnostic challenges. Clin Dermatol. 2015;33(1):90–8. A recent review of the immunology of leprosy.
Moet FJ, Pahan D, Oskam L, Richardus JH, COLEP Study Group. Effectiveness of single dose rifampicin in preventing leprosy in close contacts of patients with newly diagnosed leprosy: cluster randomised controlled trial. BMJ. 2008;336(7647):761–4.
Düppre NC, Camacho LA, da Cunha SS, Struchiner CJ, Sales AM, Nery JA, et al. Effectiveness of BCG vaccination among leprosy contacts: a cohort study. Trans R Soc Trop Med Hyg. 2008;102(7):631–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Tropical Dermatology
Rights and permissions
About this article
Cite this article
Chan, M.M.F., Smoller, B.R. Overview of the Histopathology and Other Laboratory Investigations in Leprosy. Curr Trop Med Rep 3, 131–137 (2016). https://doi.org/10.1007/s40475-016-0086-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40475-016-0086-y