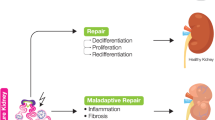
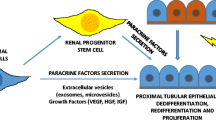
Abstract
Purpose of Review
Advances in renal regenerative medicine are crucial to address the increased rate of kidney disease. Clinicians and researchers are investigating new renal therapeutics aimed at promoting endogenous repair and/or at designing new technologies for organ and tissue regeneration. This review explores the potential promises and limitations of recent renal regenerative medicine approaches.
Recent Findings
Current research directions include efforts to delay and deter acute and chronic kidney disease progression by exploiting the immune-modulatory and tissue regenerative properties of pharmacology agents, stem cells, and extracellular vesicles. Furthermore, advancement of novel technologies in the field of xenotransplantation, and transplantable devices are showing progress towards the design of de novo replacement organs.
Summary
With integrated collaborations of multiple disciplinaries, improvements in novel technologies, and understanding of kidney disease progression, the field of renal regenerative medicine is progressing forward with promising therapeutic potential.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–72.
Hickson LJ, Eirin A, Lerman LO. Challenges and opportunities for stem cell therapy in patients with chronic kidney disease. Kidney Int. 2016;89:767–78.
Da Sacco S, Thornton ME, Petrosyan A, Lavarreda-Pearce M, Sedrakyan S, Grubbs BH, et al. Direct isolation and characterization of human nephron progenitors. Stem Cells Transl Med. 2017;6:419–33.
Lindström NO, McMahon JA, Guo J, Tran T, Guo Q, Rutledge E, et al. Conserved and divergent features of human and mouse kidney organogenesis. J Am Soc Nephrol. 2018;29:785–805.
Bonventre JV, Hurst FP, West M, Wu I, Roy-Chaudhury P, Sheldon M. A technology roadmap for innovative approaches to kidney replacement therapies: a catalyst for change. CJASN. 2019;14:1539–47.
Becherucci F, Mazzinghi B, Allinovi M, Angelotti ML, Romagnani P. Regenerating the kidney using human pluripotent stem cells and renal progenitors. Expert Opin Biol Ther. 2018;18:795–806.
Borges FT, Schor N. Regenerative medicine in kidney disease: where we stand and where to go. Pediatr Nephrol. 2018;33:1457–65.
Starke C, Betz H, Hickmann L, Lachmann P, Neubauer B, Kopp JB, et al. Renin lineage cells repopulate the glomerular mesangium after injury. J Am Soc Nephrol. 2015;26:48–54.
Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, et al. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J Am Soc Nephrol. 2006;17:2443–56.
Macconi D, Sangalli F, Bonomelli M, Conti S, Condorelli L, Gagliardini E, et al. Podocyte repopulation contributes to regression of glomerular injury induced by ACE inhibition. Am J Pathol. 2009;174:797–807.
Remuzzi A, Sangalli F, Macconi D, Tomasoni S, Cattaneo I, Rizzo P, et al. Regression of renal disease by angiotensin II antagonism is caused by regeneration of kidney vasculature. J Am Soc Nephrol. 2016;27:699–705.
Rizzo P, Novelli R, Benigni A, Remuzzi G. Inhibiting angiotensin-converting enzyme promotes renal repair by modulating progenitor cell activation. Pharmacol Res. 2016;108:16–22.
Andrianova NV, Buyan MI, Zorova LD, Pevzner IB, Popkov VA, Babenko VA, et al. Kidney cells regeneration: dedifferentiation of tubular epithelium, resident stem cells and possible niches for renal progenitors. Int J Mol Sci. 2019;20:6326.
Benigni A, Morigi M, Rizzo P, Gagliardini E, Rota C, Abbate M, et al. Inhibiting angiotensin-converting enzyme promotes renal repair by limiting progenitor cell proliferation and restoring the glomerular architecture. Am J Pathol. 2011;179:628–38.
Becher UM, Endtmann C, Tiyerili V, Nickenig G, Werner N. Endothelial damage and regeneration: the role of the renin-angiotensin-aldosterone system. Curr Hypertens Rep. 2011;13:86–92.
Barrera-Chimal J, Pérez-Villalva R, Rodríguez-Romo R, Reyna J, Uribe N, Gamba G, et al. Spironolactone prevents chronic kidney disease caused by ischemic acute kidney injury. Kidney Int. 2013;83:93–103.
Barba-Navarro R, Tapia-Silva M, Garza-Garcia C, López-Giacoman S, Melgoza-Toral I, Vázquez-Rangel A, et al. The effect of spironolactone on acute kidney injury after cardiac surgery: a randomized, placebo-controlled trial. Am J Kidney Dis. 2017;69:192–9.
Lionel L, Lechner Sebastian M, Smail M, Alan LM, Soumaya EM, Sonia P, et al. Nonsteroidal mineralocorticoid receptor antagonist finerenone protects against acute kidney injury–mediated chronic kidney disease. Hypertension. 2017;69:870–8.
Filippatos G, Anker SD, Böhm M, Gheorghiade M, Køber L, Krum H, et al. A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J. 2016;37:2105–14.
Sinha AD, Agarwal R. Clinical pharmacology of antihypertensive therapy for the treatment of hypertension in CKD. CJASN. 2019;14:757–64.
Verdoodt A, Honore PM, Jacobs R, De Waele E, Van Gorp V, De Regt J, et al. Do statins induce or protect from acute kidney injury and chronic kidney disease: an update review in 2018. J Transl Int Med. 2018;6:21–5.
•• Rota C, Morigi M, Imberti B. Stem cell therapies in kidney diseases: progress and challenges. Int J Mol Sci. 2019;20(11):2790. This review provides a thorough description of the limitation and potential of stem cell therapies in kidney disease.
Bochon B, Kozubska M, Surygała G, Witkowska A, Kuźniewicz R, Grzeszczak W, et al. Mesenchymal stem cells—potential applications in kidney diseases. Int J Mol Sci. 2019;20:2462.
Cossu G, Birchall M, Brown T, De Coppi P, Culme-Seymour E, Gibbon S, et al. Lancet commission: stem cells and regenerative medicine. Lancet. 2018;391:883–910.
Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S, et al. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27:1421–32.
Wang D, Niu L, Feng X, Yuan X, Zhao S, Zhang H, et al. Long-term safety of umbilical cord mesenchymal stem cells transplantation for systemic lupus erythematosus: a 6-year follow-up study. Clin Exp Med. 2017;17:333–40.
Reinders ME, Dreyer GJ, Bank JR, et al. Safety of allogeneic bone marrow derived mesenchymal stromal cell therapy in renal transplant recipients: the neptune study. J Transl Med. 2015;13:344.
Packham DK, Fraser IR, Kerr PG, Segal KR. Allogeneic mesenchymal precursor cells (MPC) in diabetic nephropathy: a randomized, placebo-controlled, dose escalation study. EBioMedicine. 2016;12:263–9.
Reinders MEJ, de Fijter JW, Roelofs H, Bajema IM, de Vries DK, Schaapherder AF, et al. Autologous bone marrow-derived mesenchymal stromal cells for the treatment of allograft rejection after renal transplantation: results of a phase I study. Stem Cells Transl Med. 2013;2:107–11.
Thakkar UG, Vanikar AV, Trivedi HL. Stem cell therapy: an emerging modality in glomerular diseases. Cytotherapy. 2017;19:333–48.
Swaminathan M, Stafford-Smith M, Chertow GM, Warnock DG, Paragamian V, Brenner RM, et al. Allogeneic mesenchymal stem cells for treatment of AKI after cardiac surgery. J Am Soc Nephrol. 2018;29:260–7.
Makhlough A, Shekarchian S, Moghadasali R, Einollahi B, Dastgheib M, Janbabaee G, et al. Bone marrow–mesenchymal stromal cell infusion in patients with chronic kidney disease: a safety study with 18 months of follow-up. Cytotherapy. 2018;20:660–9.
Makhlough A, Shekarchian S, Moghadasali R, Einollahi B, Hosseini SE, Jaroughi N, et al. Safety and tolerability of autologous bone marrow mesenchymal stromal cells in ADPKD patients. Stem Cell Res Ther. 2017;8(1):116.
Torres Crigna A, Daniele C, Gamez C, Medina Balbuena S, Pastene DO, Nardozi D, et al. Stem/stromal cells for treatment of kidney injuries with focus on preclinical models. Front Med (Lausanne). 2018;5:179.
Saad A, Dietz AB, Herrmann SMS, Hickson LJ, Glockner JF, McKusick MA, et al. Autologous mesenchymal stem cells increase cortical perfusion in renovascular disease. J Am Soc Nephrol. 2017;28:2777–85.
Marcheque J, Bussolati B, Csete M, Perin L. Concise reviews: stem cells and kidney regeneration: an update. Stem Cells Transl Med. 2019;8:82–92.
Caldas HC, Lojudice FH, Dias C, Fernandes-Charpiot IMM, Baptista MASF, Kawasaki-Oyama RS, et al. Induced pluripotent stem cells reduce progression of experimental chronic kidney disease but develop Wilms’ tumors. Stem Cells Int. 2017;2017:7428316.
Liu B, Ding F, Hu D, Zhou Y, Long C, Shen L, et al. Human umbilical cord mesenchymal stem cell conditioned medium attenuates renal fibrosis by reducing inflammation and epithelial-to-mesenchymal transition via the TLR4/NF-κB signaling pathway in vivo and in vitro. Stem Cell Res Ther. 2018;9:7.
Liu B, Ding F-X, Liu Y, Xiong G, Lin T, He D-W, et al. Human umbilical cord-derived mesenchymal stem cells conditioned medium attenuate interstitial fibrosis and stimulate the repair of tubular epithelial cells in an irreversible model of unilateral ureteral obstruction. Nephrology (Carlton). 2018;23:728–36.
Zheng J, Wang Q, Leng W, Sun X, Peng J. Bone marrow-derived mesenchymal stem cell-conditioned medium attenuates tubulointerstitial fibrosis by inhibiting monocyte mobilization in an irreversible model of unilateral ureteral obstruction. Mol Med Rep. 2018;17:7701–7.
Golle L, Gerth HU, Beul K, Heitplatz B, Barth P, Fobker M, et al. Bone marrow-derived cells and their conditioned medium induce microvascular repair in uremic rats by stimulation of endogenous repair mechanisms. Sci Rep. 2017;7:9444.
•• Lv L, Wu W, Feng Y, Li Z, Tang T, Liu B. Therapeutic application of extracellular vesicles in kidney disease: promises and challenges. J Cell Mol Med. 2018;22:728–37. Concise review discussing the therapeutic potential of EVs in promoting kidney repair in both acute and chronic kidney injury and further investigation needed before conducting studies in clinic.
Bruno S, Tapparo M, Collino F, Chiabotto G, Deregibus MC, Soares Lindoso R, et al. Renal regenerative potential of different extracellular vesicle populations derived from bone marrow mesenchymal stromal cells. Tissue Eng Part A. 2017;23:1262–73.
Sedrakyan S, Villani V, Da Sacco S, Tripuraneni N, Porta S, Achena A, et al. Amniotic fluid stem cell-derived vesicles protect from VEGF-induced endothelial damage. Sci Rep. 2017;7(1):16875.
Viñas JL, Spence M, Gutsol A, Knoll W, Burger D, Zimpelmann J, et al. Receptor-ligand interaction mediates targeting of endothelial colony forming cell-derived exosomes to the kidney after ischemic injury. Sci Rep. 2018;8(1):16320.
Yuan X, Li D, Chen X, Han C, Xu L, Huang T, et al. Extracellular vesicles from human-induced pluripotent stem cell-derived mesenchymal stromal cells (hiPSC-MSCs) protect against renal ischemia/reperfusion injury via delivering specificity protein (SP1) and transcriptional activating of sphingosine kinase 1 and inhibiting necroptosis. Cell Death Dis. 2017;8:3200.
Wang B, Jia H, Zhang B, Wang J, Ji C, Zhu X, et al. Pre-incubation with hucMSC-exosomes prevents cisplatin-induced nephrotoxicity by activating autophagy. Stem Cell Res Ther. 2017;8:75.
Ebrahim N, Ahmed IA, Hussien NI, Dessouky AA, Farid AS, Elshazly AM, et al Mesenchymal stem cell-derived exosomes ameliorated diabetic nephropathy by autophagy induction through the mTOR signaling pathway. Cells. 2018;7(12):226.
Eirin A, Zhu X-Y, Puranik AS, Tang H, McGurren KA, van Wijnen AJ, et al. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017;92:114–24.
Grange C, Papadimitriou E, Dimuccio V, Pastorino C, Molina J, O'Kelly R, Niedernhofer LJ, Robbins PD, Camussi G, Bussolati B. Urinary Extracellular Vesicles Carrying Klotho Improve the Recovery of Renal Function in an Acute Tubular Injury Model. Mol Ther. 2020;28(2):490-502.
Nassar W, El-Ansary M, Sabry D, Mostafa MA, Fayad T, Kotb E, et al. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater Res. 2016;20:21.
Wijkstrom M, Iwase H, Paris W, Hara H, Ezzelarab M, Cooper DKC. Renal xenotransplantation: experimental progress and clinical prospects. Kidney Int. 2017;91:790–6.
Hryhorowicz M, Zeyland J, Słomski R, Lipiński D. Genetically modified pigs as organ donors for xenotransplantation. Mol Biotechnol. 2017;59:435–44.
Niemann H, Verhoeyen E, Wonigeit K, Lorenz R, Hecker J, Schwinzer R, et al. Cytomegalovirus early promoter induced expression of hCD59 in porcine organs provides protection against hyperacute rejection. Transplantation. 2001;72:1898–906.
Iwase H, Hara H, Ezzelarab M, Li T, Zhang Z, Gao B, et al. Immunological and physiological observations in baboons with life-supporting genetically engineered pig kidney grafts. Xenotransplantation. 2017;24:e12293.
Nomura S, Ariyoshi Y, Watanabe H, Pomposelli T, Takeuchi K, Garcia G, et al. Transgenic expression of human CD47 reduces phagocytosis of porcine endothelial cells and podocytes by baboon and human macrophages. Xenotransplantation. 2020;27(1):e12549.
Fischer K, Rieblinger B, Hein R, Sfriso R, Zuber J, Fischer A, et al. Viable pigs after simultaneous inactivation of porcine MHC class I and three xenoreactive antigen genes GGTA1, CMAH and B4GALNT2. Xenotransplantation. 2020;27(1):e12560.
Kim SC, Mathews DV, Breeden CP, Higginbotham LB, Ladowski J, Martens G, et al. Long-term survival of pig-to-rhesus macaque renal xenografts is dependent on CD4 T cell depletion. Am J Transplant. 2019;19:2174–85.
Tanabe T, Watanabe H, Shah JA, Sahara H, Shimizu A, Nomura S, et al. Role of intrinsic (graft) versus extrinsic (host) factors in the growth of transplanted organs following allogeneic and xenogeneic transplantation. Am J Transplant. 2017;17:1778–90.
Heo Y, Cho Y, Oh KB, et al. Detection of pig cells harboring porcine endogenous retroviruses in non-human primate bladder after renal xenotransplantation. Viruses. 2019;11(9):801.
Niu D, Wei H-J, Lin L, George H, Wang T, Lee I-H, et al. Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9. Science. 2017;357:1303–7.
Cooper DKC, Wijkstrom M, Hariharan S, Chan JL, Singh A, Horvath K, et al. Selection of patients for initial clinical trials of solid organ xenotransplantation. Transplantation. 2017;101:1551–8.
Jagdale A, Cooper DKC, Iwase H, Gaston RS. Chronic dialysis in patients with end-stage renal disease: relevance to kidney xenotransplantation. Xenotransplantation. 2019;26:e12471.
Iwase H, Jagdale A, Yamamoto T, Zhang G, Li Q, Foote J, et al. Indicators of impending pig kidney and heart xenograft failure: relevance to clinical organ xenotransplantation - review article. Int J Surg. 2019;70:84–91.
PD Pedroso. Xenotransplantation. Nat Biotechnol 2019;37:864. https://www.nature.com/articles/s41587-019-0208-x.
Imafuku A, Oka M, Miyabe Y, Sekiya S, Nitta K, Shimizu T. Rat mesenchymal stromal cell sheets suppress renal fibrosis via microvascular protection. Stem Cells Transl Med. 2019;8:1330–41.
Hojs N, Fissell WH, Roy S. Ambulatory hemodialysis-technology landscape and potential for patient-centered treatment Clin J Am Soc Nephrol. 2020;15(1):152–159.
Gura V, Rivara MB, Bieber S, et al. A wearable artificial kidney for patients with end-stage renal disease. JCI Insight. 2016;1(8):e86397.
Salani M, Roy S, Fissell WH. Innovations in wearable and implantable artificial kidneys. Am J Kidney Dis. 2018;72:745–51.
Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157(6):1262–78.
Freedman BS. Hopes and difficulties for blastocyst complementation. Nephron. 2018;138:42–7.
Cruz NM, Freedman BS. CRISPR gene editing in the kidney. Am J Kidney Dis. 2018;71:874–83.
Ma H, Marti-Gutierrez N, Park S-W, Wu J, Lee Y, Suzuki K, et al. Correction of a pathogenic gene mutation in human embryos. Nature. 2017;548:413–9.
Trionfini P, Ciampi O, Todeschini M, Ascanelli C, Longaretti L, Perico L, et al. CRISPR-Cas9-mediated correction of the G189R-PAX2 mutation in induced pluripotent stem cells from a patient with focal segmental glomerulosclerosis. CRISPR J. 2019;2:108–20.
Cruz NM, Song X, Czerniecki SM, Gulieva RE, Churchill AJ, Kim YK, et al. Organoid cystogenesis reveals a critical role of microenvironment in human polycystic kidney disease. Nat Mater. 2017;16:1112–9.
Kim YK, Refaeli I, Brooks CR, Jing P, Gulieva RE, Hughes MR, et al. Gene-edited human kidney organoids reveal mechanisms of disease in podocyte development. Stem Cells. 2017;35:2366–78.
Boreström C, Jonebring A, Guo J, Palmgren H, Cederblad L, Forslöw A, et al. A CRISP(e)R view on kidney organoids allows generation of an induced pluripotent stem cell–derived kidney model for drug discovery. Kidney Int. 2018;94:1099–110.
Petrosyan A, Cravedi P, Villani V, Angeletti A, Manrique J, Renieri A, et al. A glomerulus-on-a-chip to recapitulate the human glomerular filtration barrier. Nat Commun. 2019;10:1–17.
Kjellstrand CM, Buoncristiani U, Ting G, Traeger J, Piccoli GB, Sibai-Galland R, et al. Short daily haemodialysis: survival in 415 patients treated for 1006 patient-years. Nephrol Dial Transplant. 2008;23:3283–9.
•• Solez K, Fung KC, Saliba KA, VLC S, Petrosyan A, Perin L, et al. The bridge between transplantation and regenerative medicine: beginning a new Banff classification of tissue engineering pathology. Am J Transplant. 2018;18:321–7. Propose a new concept that we need a new Banff classification of tissue engineering pathology to standardize and assess de novo–bioengineered solid organ transplantable success in vivo. Highlighting the need for collaboration between multiple fields to ensure advancements and success of renal regenerative medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Camille Frank and Astgik Petrosyan declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cellular Transplants
Rights and permissions
About this article
Cite this article
Frank, C.N., Petrosyan, A. Kidney Regenerative Medicine: Promises and Limitations. Curr Transpl Rep 7, 81–89 (2020). https://doi.org/10.1007/s40472-020-00273-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40472-020-00273-3