Abstract
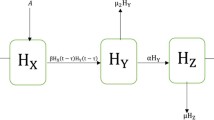
In this paper, we focus on the study of HIV–AIDS model in space and time that is adapted from the previous study in Ammi et al. (Sci Rep 12:5751, 2022) without the fractional-order derivative. The fixed controls of highly antiretroviral and immunotherapy are considered for the interaction between susceptible and infected CD4\(^+\)T cell. The equilibrium points of disease-free and endemic, positivity, boundedness and basic reproduction number of dynamical system are provided in the standard ways. For the local stability, the Fourier series is firstly employed to obtain the Jacobian matrix which is then used for further analysis of stability. Moreover, the classical numerical scheme of standard finite difference is applied to approximate our model. The stability, positivity, and consistency of numerical scheme are very important in the numerical analysis. At the last section of numerical analysis, we provide the experiments of our model numerically by varying values for the parameters of treatment HAART and Immunotherapy. We can conclude that the combinations of HAART and Immunotherapy at once are the most efficient in decreasing the infected CD4\(^+\)T cells and the treatment of immunotherapy is more effective than the treatment of HAART. Our dynamical system is eligible to predict the spread of HIV–AIDS based on the validation results with the actual data by using least square technique. Moreover, we apply the ARIMA(1,1,0) model in this paper to predict infected profile and the result has the similar trend (decreasing trend) with the HIV–AIDS model (obtained from the least square technique) and the actual data. Moreover, we employ the neural network for dynamical system, due to the significant results of best validation performance, error histogram, and regression.







Similar content being viewed by others
References
Campbell EM, Jia H, Shankar A, Hanson D, Luo W, Masciotra S, Blosser SJ (2017) Detailed transmission network analysis of a large opiate-driven outbreak of HIV infection in the United States. J Infect Dis 216(9):1053–62
Qiao YC, Xu Y, Jiang DX, Wang X, Wang F, Yang J, Wei YS (2019) Epidemiological analyses of regional and age differences of HIV/AIDS prevalence in China, 2004–2016. Int J Infect Dis 81:215–20
Paraskevis D, Nikolopoulos G, Tsiara C, Paraskeva D, Antoniadou A, Lazanas M, Hatzakis A (2011) HIV-1 outbreak among injecting drug users in Greece, 2011: a preliminary report. Eurosurveillance 16(36):19962
Doitsh G, Greene WC (2016) Dissecting how CD4 T cells are lost during HIV infection. Cell Host Microbe 19(3):280–91
Cummins NW, Badley AD (2014) Making sense of how HIV kills infected CD4 T cells: implications for HIV cure. Mol Cell Ther 2(1):20
Poorolajal J, Hooshmand E, Mahjub H, Esmailnasab N, Jenabi E (2016) Survival rate of AIDS disease and mortality in HIV-infected patients: a meta-analysis. Public Health 139:3–12
Alemu A, Yesuf A, Zerihun B, Getu M, Worku T, Bitew ZW (2020) Incidence and determinants of tuberculosis among HIV positives in Addis Ababa, Ethiopia: a Retrospective Cohort Study. Int J Infect Dis 95:59
WHO (2018) Global Tuberculosis Report Geneva
Ramírez BC, Vega YC, Shepherd BE, Le C, Turner M, Frola C, McGowan CC (2017) Outcomes of HIV-positive patients with cryptococcal meningitis in the Americas. Int J Infect Dis 63:57–63
Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, Boulware DR (2017) Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 17(8):873–81
Mekonnen Y, Hadush T, Tafere A, Tilahun A (2016) A review article on cryptosporidiosis
Sullivan MC, Rosen AO, Allen A, Benbella D, Camacho G, Cortopassi AC, Kalichman SC (2020) Falling Short of the First 90: HIV Stigma and HIV Testing Research in the 90-90-90 Era
Li D, Ma W (2007) Asymptotic properties of a HIV-1 infection model with time delay. J Math Anal Appl 335:683–91
Abdullahi YM, Nweze O (2011) A simulation of an sir mathematical model of HIV transmission dynamics using the classical Euler’s method. Shiraz Med J 12:196–202
Jiang X, Chen X, Huang T, Yan H (2021) Bifurcation and control for a predator-prey system with two delays. IEEE Trans Circuits Syst II Express Briefs 68(1):376–80
Jiang X, Chen X, Chi M, Chen J (2020) On Hopf bifurcation and control for a delay systems. Appl Math Comput 370(1):124906
Asif M, Ullah Jan S, Haider N, Mdallal QA, Jawad TA (2020) Numerical modeling of NPZ and SIR models with and without diffusion. Result Phys 19:103512
Asif M, Khan ZA, Haider N, Mdallal QA (2020) Numerical simulation for solution of SEIR models by meshless and finite difference methods. Chaos Soliton Fractals 141:110340
Hajji MA, Mdallal QA (2018) Numerical simulations of a delay model for immune system tumor interaction. Sultan Qaboos Univ J Sci 23(1):19–31
Rihan FA, Al-Mdallal QM, AlSakaji HJ, Hashish A (2019) A fractional-order epidemic model with time-delay and nonlinear incidence rate. Chaos Solitons Fractals 126:97–105
Ullah I, Ahmad S, Mdallal QA, Khan ZA, Khan H, Khan A (2020) Stability analysis of a dynamical model of tuberculosis with incomplete treatment. Adv Differ Equ. https://doi.org/10.1186/s13662-020-02950-0
Kumar S, Chauhan RP, Abdel-Aty AH, Alharthi MR (2021) A study on transmission dynamics of HIV/AIDS model through fractional operators. Results Phys 22:103855
Yu P, Zhang W (2019) Complex dynamics in a unified SIR and HIV disease model: a bifurcation theory approach. J Nonlinear Sci 29(5):2447
Dhar M, Bhattacharya P (2019) Analysis of SIR epidemic model with different basic reproduction numbers and validation with HIV and TSWV data, Iran. J Sci Technol Trans A Sci 43(5):2385
Naik PA, Zu J, Owolabi KM (2020) Global dynamics of a fractional order model for the transmission of HIV epidemic with optimal control. Chaos, Solitons Fractals 138:109826
Wang X, Wang W, Li Y (2021) Global stability of switched HIV/AIDS models with drug treatment involving caputo-fractional derivatives. Discret Dyn Nat Soc 138:109826
Babaei A, Jafari H, Liya A (2020) Mathematical models of HIV/AIDS and drug addiction in prisons. Eur Phys J Plus 135(5):1
Chang H (2021) A mathematical study on the drug resistant virus emergence with HIV/AIDS treatment cases. Heliyon 7(1):e05883
Bassey BE, Henry AO (2021) Global stability analysis of the role of antiretroviral therapy (ART) abuse in HIV/AIDS treatment dynamics. Pure Appl Math J 10(1):9
Wanduku (2020) A nonlinear multi-population behavioral model to assess the roles of education campaigns, random supply of aids, and delayed ART treatment in HIV/AIDS epidemics. Math Biosci Eng 17(6):6791
Teklu SW, Mekonnen TT (2021) HIV/AIDS-pneumonia coinfection model with treatment at each infection stage: mathematical analysis and numerical simulation. J Appl Math 2021:1–21
Kumar S, Chauhan RP, Momani S, Hadid S (2020) Numerical investigations on COVID-19 model through singular and non-singular fractional operators. Numer Methods Partial Differ Equ. https://doi.org/10.1002/num.22707
Khan MA, Ullah S, Kumar S (2021) A robust study on 2019-nCOV outbreaks through non-singular derivative. Eur Phys J Plus. https://doi.org/10.1140/epjp/s13360-021-01159-8
Kumar S, Kumar A, Samet B, Dutta H (2020) A study on fractional host-parasitoid population dynamical model to describe insect species. Numer Methods Partial Differ Equ 37:1673–1692
Mohammadi H, Kumar S, Rezapour S, Etemad S (2021) A theoretical study of the Caputo-Fabrizio fractional modeling for hearing loss due to Mumps virus with optimal control. Chaos, Solitons Fractals 144:110668
Kumar S, Kumar R, Cattani C, Samet B (2020) Chaotic behaviour of fractional predator-prey dynamical system. Chaos, Solitons Fractals 135:109811
Kumar S, Kumar R, Osman MS, Samet B (2021) A wavelet based numerical scheme for fractional order SEIR epidemic of measles by using Genocchi polynomials. Numer Methods Partial Differ Equ 37:1250–1268
Ammi MRS, Tahiri M, Tilioua M, Zeb A, Khan I, Andualem M (2022) Global analysis of a time fractional order spatio-temporal SIR model. Sci Rep 12:5751
Fraser C, Donnelly CA, Cauchemez S et al (2009) Pandemic potential of a strain of influenza A (H1N1): early findings. Science 324:1557–1561
Chakrabrty A, Singh M, Lucy B, Ridland P (2007) Predator-prey model with pry-taxis and diffusion. Math Comput Model 46:482–98
Ahmed N, Tahira SS, Rafiq M, Rehman MA, Ali M, Ahmad MO (2019) Positivity preserving operator splitting nonstandard finite difference methods for SEIR reaction diffusion model. Open Math 17:313–30
Lin Z, Pederson M (2004) Stability in a di usive food-chain model with Michaelis-Menten functional response. Nonlinear Anal 57:421–433
Ahmed N, Rafiq M, Rehman MA, Iqbal MS, Ali M (2019) Numerical modeling of three dimensional Brusselator system. AIP Adv 09(01):01–19
Charpentier BMC, Kojouharov HV (2013) Unconditionally positive preserving scheme for advection-diffusion-reaction equations. Math Comput Model 57(10):2177–1285
Fujimoto T, Ranade R (2004) Two characterizations of inverse positive matrices: the Hawkins-Simon condition and the le chatelier-braun principle. Electron J Linear Algebra ELA 11(1):01–18
Smith GD (1985) Numerical solution of partial differential equations: finite difference methods, 3rd edn. Clarendon Press, Oxford
Mitchell AR, Griffiths DF (1980) Finite difference methods in partial differential equations. T. Wiley, Hoboken
Chinviriyasit S, Chinviriyasit W (2010) Numerical modelling of an SIR epidemic model with diffusion. Appl Math Comput 216:395–409
Kustiawan C (2018) A Numerical scheme for a reaction diffusion equation with time delay and impulses. Far East J Math Sci 106(2):451–62
Ahmed N, Elsonbaty A, Adel W, Baleanu D, Rafiq M (2020) Stability analysis and numerical simulations of spatiotemporal HIV CD4+ T cell model with drug therapy. Chaos 30:083122
Samsuzzoha M, Singh M, Lucy D (2013) Parameter estimation of influenza epidemic model. Appl Math Comput 220:616
Kristensen MR (2014) Parameter estimation in nonlinear dynamical systems Master’s Thesis. Technical University of Denmark, Kongens
Funding
Mohammad Ghani received financial support for the research under Contract Number 121/UN3.1.17/PT/2022 by the Faculty of Advanced Technology and Multidiscipline, Universitas Airlangga, Indonesia.
Author information
Authors and Affiliations
Contributions
MG contributed to formal analysis, investigation, methodology, software, writing—original draft, and writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper. It is to specifically state that “No Competing interests are at stake and there is No Conflict of Interest” with other people or organizations that could inappropriately influence or bias the content of the paper.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghani, M. Dynamics of spatio-temporal HIV–AIDS model with the treatments of HAART and immunotherapy. Int. J. Dynam. Control 12, 1366–1391 (2024). https://doi.org/10.1007/s40435-023-01284-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40435-023-01284-5
Keywords
- HIV–AIDS model
- Standard finite difference
- Highly active antiretroviral therapy
- Immunotherapy
- Basic reproduction number
- Least square technique
- Disease transmissions
- Neural network