Abstract
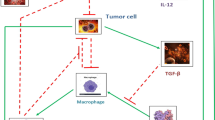
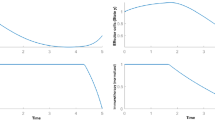
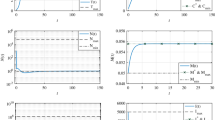
In this work, a nominal-plus-neighboring-optimal control approach is suggested for the treatment of cancer using the adoptive cellular immunotherapy. The main goal of this therapy is to minimize both tumor concentration and treatment costs while restoring natural defense mechanisms and activating immune response. In the presence of an additional initial concentration of cancer cells, the biological effects of the introduction of a neighboring-optimal treatment in addition to the existing nominally therapy are explored and investigated. The optimal control problem is presented by defining appropriate objective functions. The Pontryagin’s maximum principle and the Pontryagin procedure are both used to obtain optimal solutions for subsequently providing nominal and neighboring-optimal control configurations. The optimal systems are derived and solved numerically using an adapted iterative method with a Runge–Kutta fourth order scheme.















Similar content being viewed by others
References
Chelur DS, Chalfie M (2007) Targeted cell killing by reconstituted caspases. Proc Natl Acad Sci 104(7):2283–2288
Martin RB (1992) Optimal control drug scheduling of cancer chemotherapy. Automatica 28(6):1113–1123
Swan GW (1990) Role of optimal control theory in cancer chemotherapy. Math Biosci 101(2):237–284
Engelhart M, Lebiedz D, Sager S (2011) Optimal control for selected cancer chemotherapy ODE models: a view on the potential of optimal schedules and choice of objective function. Math Biosci 229(1):123–134
Zouhri S, Saadi S, Elmouki I, Hamdache A, Rachik M (2013) Mixed immunotherapy and chemotherapy of tumors: optimal control approach. Int J Comput Sci Issues 10(4):1
Bomford CK, Kunkler IH (1993) Walter and Miller’s textbook of radiotherapy: radiation physics, therapy, and oncology. Churchill Livingstone, London
Castiglione F, Piccoli B (2007) Cancer immunotherapy, mathematical modeling and optimal control. J Theor Biol 247(4):723–732
De Pillis LG, Gu W, Radunskaya AE (2006) Mixed immunotherapy and chemotherapy of tumors: modeling, applications and biological interpretations. J Theor Biol 238(4):841–862
Kirschner D, Panetta JC (1998) Modeling immunotherapy of the tumorimmune interaction. J Math Biol 37(3):235–252
Chan C, George AJ, Stark J (2003) T cell sensitivity and specificity-kinetic proofreading revisited. Discrete Contin Dyn Syst Ser B 3(3):343–360
Blattman JN, Greenberg PD (2004) Cancer immunotherapy: a treatment for the masses. Science 305(5681):200–205
Starkov KE, Krishchenko AP (2014) On the global dynamics of one cancer tumour growth model. Commun Nonlinear Sci Numer Simul 19(5):1486–1495
Beerenwinkel N, Schwarz RF, Gerstung M, Markowetz F (2015) Cancer evolution: mathematical models and computational inference. Syst Biol 64(1):e1–e25
Babaei N, Salamci MU (2014) State dependent riccati equation based model reference adaptive stabilization of nonlinear systems with application to cancer treatment. In: Proceedings of the 19th IFAC World Congress, Cape Town, South Africa
Babaei N, Salamci MU (2015) Personalized drug administration for cancer treatment using model reference adaptive control. J Theor Biol 371:24–44
Swanson KR, Bridge C, Murray JD, Alvord EC (2003) Virtual and real brain tumors: using mathematical modeling to quantify glioma growth and invasion. J Neurol Sci 216(1):1–10
Bunimovich-Mendrazitsky S, Shochat E, Stone L (2007) Mathematical model of BCG immunotherapy in superficial bladder cancer. Bull Math Biol 69(6):1847–1870
Elmouki I, Saadi S (2014) BCG immunotherapy optimization on an isoperimetric optimal control problem for the treatment of superficial bladder cancer. Int J Dyn Control 1–7. doi:10.1007/s40435-014-0106-5
Saadi S, Elmouki I, Hamdache A (2015) Impulsive control dosing BCG immunotherapy for non-muscle invasive bladder cancer. Int J Dyn Control 3(3):313–323
Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, Frohlich MW (2009) Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer 115(16):3670–3679
Nazari M, Ghaffari A (2015) The effect of finite duration inputs on the dynamics of a system: proposing a new approach for cancer treatment. Int J Biomath 8(03):1550036
Nazari M, Ghaffari A, Arab F (2015) Finite duration treatment of cancer by using vaccine therapy and optimal chemotherapy: state-dependent Riccati equation control and extended Kalman filter. J Biol Syst 23(01):1–29
Ghaffari A, Nazari M, Arab F (2015) Suboptimal mixed vaccine and chemotherapy in finite duration cancer treatment: state-dependent Riccati equation control. J Braz Soc Mech Sci Eng 37(1):45–56
Sahami F, Salamci MU (2015) Decentralized model reference adaptive control design for nonlinear systems; state dependent Riccati equation approach. In: 2015 16th international Carpathian control conference (ICCC), IEEE, pp 437–442
Cimen T (2008) State-dependent Riccati equation (SDRE) control: a survey. In: Proceedings of the 17th World Congress of the international federation of automatic control (IFAC). Seoul, Korea, July, pp 6–11
Naidu DS (2002) Optimal control systems, vol 2. CRC Press, Boca Raton
Dutcher J (2002) Current status of interleukin-2 therapy for metastatic renal cell carcinoma and metastatic melanoma. Oncology (Williston Park) 16(11 Suppl 13):4–10
Hamdache A, Saadi S, Elmouki I, Zouhri S (2013) Two therapeutic approaches for the treatment of HIV infection in AIDS stage. Appl Math Sci 7(105):5243–5257
Rosenberg SA (2008) Overcoming obstacles to the effective immunotherapy of human cancer. Proc Natl Acad Sci 105(35):12643–12644
Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME (2008) Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer 8(4):299–308
Rosenberg SA, Yang JC, Restifo NP (2004) Cancer immunotherapy: moving beyond current vaccines. Nat Med 10(9):909–915
Zitvogel L, Kroemer G (2008) Introduction: the immune response against dying cells. Curr Opin Immunol 20(5):501–503
Hamdache A, Elmouki I, Saadi S (2014) Optimal control with an isoperimetric constraint applied to cancer immunotherapy. Int J Comput Appl 94(15):31–37
Burden TN, Ernstberger J, Fister KR (2004) Optimal control applied to immunotherapy. Discrete Contin Dyn Syst Ser B 4(1):135–146
Ben-Ami E, Schachter J (2015) Adoptive transfer of tumor-infiltrating lymphocytes for melanoma: new players, old game. Immunotherapy 7(5):477–479
Stengel RF, Ghigliazza RM, Kulkarni NV (2002) Optimal enhancement of immune response. Bioinformatics 18(9):1227–1235
Fleming W, Rishel R (1975) Deterministic and stochastic optimal control. Springer, New York
Pontryagin LS (1987) Mathematical theory of optimal processes. CRC Press, Boca Raton
Stengel RF (2012) Optimal control and estimation. Courier Corporation, North Chelmsford
Lenhart S, Workman JT (2007) Optimal control applied to biological models. CRC Press, Boca Raton
McAsey M, Mou L, Han W (2012) Convergence of the forward-backward sweep method in optimal control. Comput Optim Appl 53(1):207–226
Graves RN (2010) A method to accomplish the optimal control of continuous dynamical systems with impulse controls via discrete optimal control and utilizing optimal control theory to explore the emergence of synchrony
Brugnano L, Iavernaro F, Trigiante D (2015) Analysis of Hamiltonian boundary value methods (HBVMs): a class of energy-preserving Runge–Kutta methods for the numerical solution of polynomial Hamiltonian systems. Commun Nonlinear Sci Numer Simul 20(3):650–667
Kirschner D, Tsygvintsev A (2009) On the global dynamics of a model for tumor immunotherapy. Math Biosci Eng 6(3):573–583
Starkov KE, Coria LN (2013) Global dynamics of the Kirschner–Panetta model for the tumor immunotherapy. Nonlinear Anal Real World Appl 14(3):1425–1433
Banerjee S (2008) Immunotherapy with interleukin-2: a study based on mathematical modeling. Int J Appl Math Comput Sci 18(3):389–398
Lukes DL (1982) Differential equations. Elsevier, Amsterdam
Elmouki I, Saadi S (2015) Quadratic and linear controls developing an optimal treatment for the use of BCG immunotherapy in superficial bladder cancer. Optim Control Appl Methods . doi:10.1002/oca.2161
Trelat E (2005) Contrôle optimal: théorie et applications. Vuibert, Paris
Meyer GH (1973) Initial value methods for boundary value problems. Academic Press, New York
Ramirez WF (1994) Process control and identification. Academic Press, New York
Cheney E, Kincaid D (2012) Numerical mathematics and computing. Cengage Learning, Boston
Zill D, Wright W (2012) Differential equations with boundary-value problems. Cengage Learning, Boston
Grewal MS, Andrews AP (2011) Kalman filtering: theory and practice using MATLAB. Wiley, New York
Xue D, Chen Y (2008) Solving applied mathematical problems with MATLAB. CRC Press, Boca Raton
Siddiqui I, Mantovani A, Allavena P (2015) Adoptive T-cell therapy: optimizing chemokine receptor-mediated homing of T cells in cancer immunotherapy. In: Rezaei N (ed) Cancer immunology. Bench to bedside immunotherapy of cancers. Springer, Berlin, pp 263–282
Darcy PK, Neeson PJ (2015) Adoptive immunotherapy: a new era for the treatment of cancer. Immunotherapy 7(5):469–471
Rosenberg SA, Restifo NP (2015) Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348(6230):62–68
Stefanovic S, Schuetz F, Sohn C, Beckhove P, Domschke C (2014) Adoptive immunotherapy of metastatic breast cancer: present and future. Cancer Metastasis Rev 33(1):309–320
Shindo Y, Hazama S, Maeda Y, Matsui H, Iida M, Suzuki N, Oka M (2014) Adoptive immunotherapy with MUC1-mRNA transfected dendritic cells and cytotoxic lymphocytes plus gemcitabine for unresectable pancreatic cancer. J Transl Med 12:175
Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Rosenberg SA (2005) Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol 23(10):2346–2357
Shaffer DR, Cruz CRY, Rooney CM (2013) Adoptive T cell transfer. In: Curiel TJ (ed) Cancer immunotherapy. Paradigms, practice and promise. Springer, New York, pp 47–70
Acknowledgments
The authors would like to thank the Editor in Chief and all anonymous referees for their valuable comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamdache, A., Saadi, S. & Elmouki, I. Nominal and neighboring-optimal control approaches to the adoptive immunotherapy for cancer. Int. J. Dynam. Control 4, 346–361 (2016). https://doi.org/10.1007/s40435-015-0205-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40435-015-0205-y
Keywords
- Adoptive cellular immunotherapy
- Pontryagin’s maximum principle
- State-dependent Riccati equation
- Neighboring-optimal control problem
- Forward backward sweep method