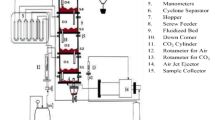
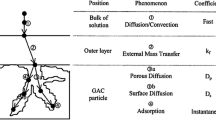
Abstract
An inverse approach (IA) was used to estimate unknown physical parameters selected of a mathematical model built up (MMB) for the adsorption process of CO2 on an activated carbon (AC) bed in a fixed-bed adsorber (FBA). The MMB was reported as the direct problem (DP) for the FBA and is used as the working model of this article. The DP is defined for mass balance equations of CO2 in gaseous and solid phases inside the FBA. The implicit finite volume method and explicit finite difference method were used to solve the DP of this work. These methods were used to test the better method regarding to processing time of the DP. The IA has been applied to the DP to adjust the axial dispersion coefficient of CO2, gas–solid mass transfer coefficient, and adsorption constant of CO2 on the AC bed of the FBA using the Levenberg–Marquardt (LM) method. The solution procedures applied in this work for direct and inverse problems have increased the reliability of the estimation of the chosen parameters from the DP. The results of this work show that the LM method was successfully applied to an adsorption problem to estimate the parameters chosen of the MMB. The numerical simulations have reported that the MMB predicts concentration distribution of CO2 in the gaseous and solid phases, and therefore, the simulated results enable us further generalization of the MMB to adsorption of CO2 on the AC inside the FBA.






Similar content being viewed by others
Abbreviations
- \(\mathrm{AC}\) :
-
Activated carbon
- \(\mathrm{CCS}\) :
-
Carbon capture and storage
- \(\mathrm{CI}\) :
-
Confidence interval
- \(\mathrm{CPDEs}\) :
-
Coupled partial differential equations
- \(\mathrm{GSHMM}\) :
-
Gas–solid heterogeneous mathematical model
- \(\mathrm{DP}\) :
-
Direct problem
- \(\mathrm{DAE}\) :
-
Discretized algebraic equation
- \(\mathrm{EFD}\) :
-
Explicit finite difference
- \(\mathrm{ES}\) :
-
Experimental system
- \(\mathrm{FBA}\) :
-
Fixed-bed adsorber
- \(\mathrm{GD}\) :
-
Gradient descent
- \(\mathrm{GHGs}\) :
-
Greenhouse gases
- \(\mathrm{GN}\) :
-
Gauss–Newton
- \(\mathrm{IA}\) :
-
Inverse approach
- \(\mathrm{IFV}\) :
-
Implicit finite volume
- \(\mathrm{LI}\) :
-
Langmuir isotherm
- \(\mathrm{LM}\) :
-
Levenberg–Marquardt
- \(\mathrm{MMB}\) :
-
Mathematical model built up
- \(\mathrm{MRD}\) :
-
Maximum relative deviation
- \(\mathrm{NAEs}\) :
-
Nonlinear algebraic equations
- \(\mathrm{NLAI}\) :
-
Nonlinear adsorption isotherm
- \(\mathrm{NR}\) :
-
Newton–Raphson
- \(\mathrm{PDEs}\) :
-
Partial differential equations
- \(\mathrm{RMSE}\) :
-
Root mean square error (–)
- \(\mathrm{SO}\) :
-
Simultaneous optimization
- \(\mathrm{SQR}\) :
-
Objective function
- \(\mathrm{UA}\) :
-
Uncertainty analysis
- \(C_{{\text{g}}}\) :
-
Concentration of CO2 in the gas phase \(\left( {{\text{mol|m}}^{3} } \right)\)
- \(C_{{\text{g,0}}}\) :
-
Feed concentration of CO2 in the gas phase \(\left( {{\text{mol|m}}^{3} } \right)\)
- \(C_{{\text{p}}}\) :
-
Concentration of CO2 in the solid phase \(\left( {{\text{mol|m}}^{3} } \right)\)
- \(D_{{{\text{ax}}}}\) :
-
Axial dispersion coefficient of CO2 \(\left( {{\text{m}}^{2} {\text{|min}}} \right)\)
- \(d_{{\text{c}}}\) :
-
Absorber diameter \(\left( {\text{m}} \right)\)
- \(D_{{{\text{CO}}_{2} - {\text{N}}_{2} }}\) :
-
Binary molecular diffusivity \(\left( {{\text{m}}^{2} {\text{|min}}} \right)\)
- \(D_{{{\text{m}},{\text{CO}}_{2} }}\) :
-
Mixture molecular diffusion coefficient \(\left( {{\text{m}}^{2} {\text{|min}}} \right)\)
- \(D_{{{\text{k}},{\text{CO}}_{2} }}\) :
-
Knudsen diffusivity \(\left( {{\text{m}}^{2} {\text{|min}}} \right)\)
- \(J\left( {p_{j} } \right)\) :
-
Jacobian or sensitivity matrix \(\left( {j = 1, 2, \ldots , N} \right)\)
- \(J_{ij}\) :
-
Sensitivity coefficients \(\left( {i = 1,2, \ldots , M;j = 1, 2, \ldots , N} \right)\)
- \(d_{{\text{p}}}\) :
-
Particle average diameter \(\left( {{\text{mm}}} \right)\)
- \(d_{{\text{c}}}\) :
-
Inner diameter (m)
- \(D_{{{\text{p}},{\text{CO}}_{2} }}\) :
-
Effective pore diffusion coefficient of CO2 \(\left( {{\text{m}}^{2} {\text{|min}}} \right)\)
- \(d_{{{\text{pore}}}}\) :
-
Diameter of the pores (μm)
- \({K}_{{\mathrm{CO}}_{2}}\) :
-
Adsorption constant of CO2 \(\left({\mathrm{m}}^{3}|\mathrm{mol}\right)\)
- \({k}_{\mathrm{gs}}\) :
-
Gas–solid mass transfer coefficient \(\left(\mathrm{m}|\mathrm{min}\right)\)
- \({k}_{\mathrm{gs},\mathrm{eff}}\) :
-
Effective mass transfer coefficient \(\left(\mathrm{m}|\mathrm{min}\right)\)
- \(L\) :
-
Bed length \(\left(\mathrm{m}\right)\)
- \({M}_{{\mathrm{CO}}_{2}}\) :
-
Molecular weight of CO2 \(\left(\mathrm{kg}|\mathrm{mol}\right)\)
- \({M}_{{\mathrm{N}}_{2}}\) :
-
Molecular weight of component j \(\left(\mathrm{kg}|\mathrm{mol}\right)\)
- \({n}_{\mathrm{p}}\) :
-
Point numbers (–)
- \({P}_{\mathrm{op}.}\) :
-
Operating pressure (kPa)
- \({p}_{j}\) :
-
Unknown parameters to be estimated \(\left(j=1, 2, \dots , N\right)\)
- \({Q}_{\mathrm{g}}\) :
-
Gas flow rates \(\left({\mathrm{m}}^{3}|\mathrm{min}\right)\)
- \({q}_{\mathrm{max}}\) :
-
Maximum amount adsorbed of CO2 in the solid phase \(\left(\mathrm{mol}|\mathrm{kg}\right)\)
- \({q}_{\mathrm{p}}\) :
-
Absolute amount adsorbed of CO2 in the solid phase \(\left(\mathrm{mol}|\mathrm{kg}\right)\)
- \(R\) :
-
Radial coordinate (m)
- \({R}^{2}\) :
-
Determination coefficient (–)
- \({R}_{\mathrm{gas}}\) :
-
Gas constant (J mol−1 K−1)
- \({S}_{\mathrm{p}}\) :
-
Specific surface area (m2 g−1)
- \(t\) :
-
Time (min)
- \({T}_{\mathrm{op}.}\) :
-
Operating temperature (K)
- \({V}_{\mathrm{sp}}\) :
-
Superficial gas velocity (m min−1)
- \(z\) :
-
Spatial coordinate (m)
- \({\varepsilon }_{\mathrm{b}}\) :
-
Bed porosity \(\left({\mathrm{m}}^{3}\mathrm{ gas}/{\mathrm{m}}^{3}\mathrm{ absorber}\right)\)
- \({\varepsilon }_{\mathrm{p}}\) :
-
Particle porosity \(\left({\mathrm{m}}^{3}\mathrm{ pores}/{\mathrm{m}}^{3}\mathrm{ particle}\right)\)
- \({\varepsilon }_{{\mathrm{CO}}_{2}-{\mathrm{N}}_{2}}\) :
-
Binary characteristic energy of molecules (–)
- \({\varepsilon }_{{\mathrm{CO}}_{2}}\) :
-
Characteristic energy of CO2 (–)
- \({\varepsilon }_{{\mathrm{N}}_{2}}\) :
-
Characteristic energy of N2 (–)
- \({\rho }_{\mathrm{p}}\) :
-
Density of AC particles (kgcat m−3)
- \({\sigma }_{\mathrm{m}}\) :
-
Standard deviation of the measurement errors
- \({\sigma }_{{\mathrm{CO}}_{2}-{\mathrm{N}}_{2}}\) :
-
Binary characteristic length of molecules (–)
- \({\sigma }_{{\mathrm{CO}}_{2}}\) :
-
Characteristic Lennard–Jones length of CO2 (–)
- \({\sigma }_{{\mathrm{N}}_{2}}\) :
-
Characteristic Lennard–Jones length of N2 (–)
- \({\mu }^{\rm k}\) :
-
Positive scalar called relaxation parameter
- \({\Psi }^{\rm k}\) :
-
Diagonal matrix
- \({\tau }_{\mathrm{p}}\) :
-
Pore tortuosity (–)
- \({\chi }^{2}\) :
-
Chi-square (–)
- \({\Omega }_{{\mathrm{CO}}_{2}-{\mathrm{N}}_{2}}\) :
-
Binary diffusion collision integral (–)
- \(\mathrm{ax}\) :
-
Axial
- \({\mathrm{CO}}_{2}\) :
-
Carbon dioxide
- CO2 − N2 :
-
Carbon dioxide–nitrogen
- \(\mathrm{g}\) :
-
Gas
- \(\mathrm{g},0\) :
-
Feed
- \(\mathrm{gs}\) :
-
Solid–gas
- \(\mathrm{gs},\mathrm{eff}\) :
-
Effective solid–gas
- \(ij\) :
-
Row and column
- \(i\) :
-
Row
- \(j\) :
-
Column
- \(\mathrm{k},{\mathrm{CO}}_{2}\) :
-
Knudsen of CO2
- \(\mathrm{m}\) :
-
Molecular
- \(\mathrm{m},{\mathrm{CO}}_{2}\) :
-
Mass of CO2
- \(\mathrm{max}\) :
-
Maximum
- \({\mathrm{N}}_{2}\) :
-
Nitrogen
- \(\mathrm{op}\) :
-
Operating
- \(\mathrm{p}\) :
-
Particle
- \(\mathrm{pore}\) :
-
Pore identification
- \(\mathrm{p},{\mathrm{CO}}_{2}\) :
-
Pore effective
- \(\mathrm{sp}\) :
-
Superficial
- Reg :
-
Reynolds number, \({\mathrm{Re}}_{\mathrm{g}}= \frac{{\rho }_{\mathrm{g}}{V}_{\mathrm{sg }}{d}_{\mathrm{p}}}{{\mu }_{\mathrm{g}}}\)
- Scg, i :
-
Schmidt number, \({\mathrm{Sc}}_{\mathrm{g},i}= \frac{{\mu }_{\mathrm{g}}}{{\rho }_{\mathrm{g}}{D}_{\mathrm{m}}}\)
References
Pal A, Rocky KA, Saha B (2021) Thermodynamic analysis of promising biomass-derived activated carbons/CO2 based adsorption cooling systems. J CO2 Util 46:101457. https://doi.org/10.1016/j.jcou.2021.101457
Chen SJ, Fu Y, Huang YX, Tao ZC, Zhu M (2016) Experimental investigation of CO2 separation by adsorption methods in natural gas purification. Appl Energy 179:329–337. https://doi.org/10.1016/j.apenergy.2016.06.146
Qasem NAA, Ben-Mansour R, Habib AM (2018) An efficient CO2 adsorptive storage using MOF-5 and MOF-177. Appl Energy 210:317–326. https://doi.org/10.1016/j.apenergy.2017.11.011
Zhu X, Shi Y, Cai N (2016) Integrated gasification combined cycle with carbon dioxide capture by elevated temperature pressure swing adsorption. Appl Energy 176:196–208. https://doi.org/10.1016/j.apenergy.2016.05.068
Mohan M, Sharma VK, Kumar EA, Gayathri V (2019) Hydrogen storage in carbon materials-a review. Energy Storage 35:1–26. https://doi.org/10.1002/est2.35
Durán I, Rubiera F, Pevida C (2022) Modeling a biogas upgrading PSA unit with a sustainable activated carbon derived from pine sawdust. Sensitivity analysis on the adsorption of CO2 and CH4 mixtures. Chem Eng J 428:132564. https://doi.org/10.1016/j.cej.2021.132564
Dantas TLP, Luna FMT, Silva JI Jr, Azevedo DCS, Grande CA, Rodrigues AE (2011) Carbon dioxide-nitrogen separation through adsorption on activated carbon in a fixed bed. Chem Eng J 169:11–19. https://doi.org/10.1016/j.cej.2010.08.026
Perry RJ, O’Brien MJ (2011) Amino disiloxanes for CO2 capture. Energy Fuels 25:1906–1918. https://doi.org/10.1021/ef101564h
Kongnoo A, Tontisirin S, Worathanakul P, Phalakornkule C (2017) Surface characteristics and CO2 adsorption capacities of acid-activated zeolite 13X prepared from palm oil mill fly ash. Fuel 193:385–394. https://doi.org/10.1016/j.fuel.2016.12.087
Naidu H, Mathews AP (2019) Acetic acid adsorption dynamics in stratified tapered beds. Chem Eng J 371:337–347. https://doi.org/10.1016/j.cej.2019.04.034
Dias VF, Silva JD (2022) An experimental investigation of gas-liquid-solid transfer and external wetting efficiency on open-cell foam in a three-phase packed bed reactor: validation and parameter estimation. Braz J Chem Eng 38:1–18. https://doi.org/10.1007/s43153-021-00217-z
Cruz BM, Silva JD (2017) A two-dimensional mathematical model for the catalytic steam reforming of methane in both conventional fixed-bed and fixed-bed membrane reactors for the Production of hydrogen. Int J Hydrog Ener 42:23670–23690. https://doi.org/10.1016/j.ijhydene.2017.03.019
Silva JD, Abreu CAM (2016) Modelling and simulation in conventional fixed-bed and fixed-bed membrane reformers for the steam reforming of methane. Int J Hydrog Energy 41:11660–11674. https://doi.org/10.1016/j.ijhydene.2016.01.083
Dias VF, Silva JD (2020) Mathematical modelling of the solar - driven steam reforming of methanol for a solar thermochemical micro - fluidized bed reformer: thermal performance and thermochemical conversion. J Braz Soc Mech Sci Eng 42:447. https://doi.org/10.1007/s40430-020-02529-6
Lima KPM, Dias VF, Silva JD (2020) Numerical modelling for the solar driven bi-reforming of methane for the production of syngas in a solar thermochemical micro-packed bed reactor. Int J Hydrog Energy 45:10353–10369. https://doi.org/10.1016/j.ijhydene.2019.08.241
Medeiros JPF, Dias VF, Silva JM, Silva JD (2021) Thermochemical performance analysis of the steam reforming of methane in a fixed bed membrane reformer: a modelling and simulation study. Membranes 11(6):1–26. https://doi.org/10.3390/membranes11010006
Silva JD (2012) Dynamic modelling for a trickle-bed reactor using the numerical inverse Laplace transform technique. Proc Eng 42:454–470. https://doi.org/10.1016/j.proeng.2012.07.437
Silva JD, Abreu CAM (2012) Mathematical modeling of a three phase trickle bed reactor. Braz J Chem Eng 29:567–576. https://doi.org/10.1590/S0104-66322012000300014
Silva PBA, Carvalho JDCG, Silva JD (2019) Hydrogen adsorption on Ni/γ-Aℓ2O3 in a fixed-bed adsorber: experimental validation and numerical modelling. Int J Hydrog Energy 44:304–317. https://doi.org/10.1016/j.ijhydene.2018.07.203
Anjos EB, Silva Filho AM, Silva JD (2020) Numerical simulation of the steam reforming of toluene in a fixed-bed catalytic reformer to produce hydrogen. J Braz Soc Mech Sci Eng 42:114. https://doi.org/10.1007/s40430-020-2195-8
Rios RB, Correia LS, Bastos-Neto M, Torres AEB, Hatimondi SA, Ribeiro AM, Rodrigues AE, Cavalcante CL Jr, de Azevedo DCS (2014) Evaluation of carbon dioxide-nitrogen separation through fixed bed measurements and simulations. Adsorption 20:945–957. https://doi.org/10.1007/s10450-014-9639-3
Mohamadinejad H, Knox JC, Smith JE (2003) Experimental and numerical investigation of two-dimensional CO2 adsorption/desorption in packed sorption beds under non-ideal flows. Sep Sci Tech 38:3875–3904. https://doi.org/10.1081/SS-120024710
Figueroa JD, Fout T, Plasynski S, McIlvried H, Srivastava RD (2018) Advances in CO2 capture technology-the U.S. Department of energy’s carbon sequestration program. Int J Greenh Gas Control 2:9–20. https://doi.org/10.1016/S1750-5836(07)00094-1
Gray ML, Champagne KJ, Fauth D, Baltrus JP, Pennline H (2008) Performance of immobilized tertiary amine solid sorbents for the capture of carbon dioxide. Int J Greenh Gas Control 2:3–8. https://doi.org/10.1016/S1750-5836(07)00088-6
Ogretim E, Mulkeen E, Gray D, Bromhal GS (2012) A parametric study of the transport of CO2 in the near-surface. Int J Greenh Gas Control 9:294–302. https://doi.org/10.1016/j.ijggc.2012.04.007
Hu Y, Liu X, Zhou Z, Liu W, Xu M (2017) Pelletization of MgO-based sorbents for intermediate temperature CO2 capture. Fuel 187:328–337. https://doi.org/10.1016/j.fuel.2016.09.066
Joss L, Gazzani M, Mazzotti M (2017) Rational design of temperature swing adsorption cycles for post-combustion CO2 capture. Chem Eng Sci 158:709–716. https://doi.org/10.1016/j.ces.2016.10.013
Silva JD, Oliveira CB (2013) Mathematical modelling for the adsorption process CO2 in nanopores of catalytic particles in a fixed bed reactor using numeral Laplace transform. Chem Eng Trans 35:829–835. https://doi.org/10.3303/CET1335138
Mulgundmath VP, Tezel FH, Thibault J (2012) Fixed bed adsorption for the removal of carbon dioxide from nitrogen: breakthrough behaviour and modelling for heat and mass transfer. Sep Purif Technol 85:17–27. https://doi.org/10.1016/j.seppur.2011.07.038
Phromprasit J, Powell J, Assabumrungrat S (2016) Metals (Mg, Sr and Al) modified CaO based sorbent for CO2 sorption/desorption stability in fixed bed reactor for high temperature application. Chem Eng J 284:1212–1223. https://doi.org/10.1016/j.cej.2015.09.038
Casas N, Schell J, Pini R, Mazzotti M (2012) Fixed bed adsorption of CO2/H2 mixtures on activated carbon: experiments and modelling. Adsorption 18:143–161. https://doi.org/10.1007/s10450-012-9389-z
Chang AC, Chuang SC, Gray M, Soong Y (2003) In-situ infrared study of CO2 adsorption on SBA-15 grafted with γ-(Aminopropyl) triethoxysilane. Energy Fuels 17:468–473. https://doi.org/10.1021/ef020176h
Drage TC, Snape CF, Stevens LA, Wood J, Wang J, Cooper AI, Dawson R, Guo X, Satterley C, Irons R (2012) Materials challenges for the development of solid sorbents for post-combustion carbon capture. J Mater Chem 22:2815–2823. https://doi.org/10.1039/C2JM12592G
Li H, Haas-Santo K, Schygulla U, Dittmeyer R (2015) Inorganic microporous membranes for H2 and CO2 separation-review of experimental and modeling progress. Chem Eng Sci 127:401–417. https://doi.org/10.1016/j.ces.2015.01.022
Liu B, Finkel M, Grathwohl P (2022) First order approximation for coupled film and intraparticle pore diffusion to model sorption/desorption batch experiments. J Hazard Mater 429:128314. https://doi.org/10.1016/j.jhazmat.2022.128314
Babatunde KA, Negash BM, Jufar SR, Ahmed TY, Mojid MR (2022) Adsorption of gases on heterogeneous shale surfaces: a review. J Pet Sci Eng 208:109466. https://doi.org/10.1016/j.petrol.2021.109466
Melouki R, Ouadah A, Llewellyn PL (2020) The CO2 adsorption behavior study on activated carbon synthesized from olive waste. J CO2 Util 42:101292. https://doi.org/10.1016/j.jcou.2020.101292
Wawrzyńczak D, Panowski M, Majchrzak-Kucęba I (2019) Possibilities of CO2 purification coming from oxy-combustion for enhanced oil recovery and storage purposes by adsorption method on activated carbon. Energy 180:787–796. https://doi.org/10.1016/j.energy.2019.05.068
Lucas S, Calvo MP, Palencia C, Cocero MJ (2004) Mathematical model of supercritical CO2 adsorption on activated carbon effect of operating conditions and adsorption scale-up. J Supercrit Fluids 32:193–201. https://doi.org/10.1016/j.supflu.2004.02.008
Chena C, Wha-Seung A (2014) CO2 adsorption on LTA zeolites: effect of mesoporosity. Appl Surf Sci 311:107–109. https://doi.org/10.1016/j.apsusc.2014.04.218
Serna-Guerrero R, Belmabkhout Y, Sayari A (2010) Modeling CO2 adsorption on amine-functionalized mesoporous silica: 1. A semi-empirical equilibrium model. Chem Eng J 161:173–181. https://doi.org/10.1016/j.cej.2010.04.024
Silva MSP, Mota JPB, Rodrigues AE (2012) Fixed-bed adsorption of aromatic C8 isomers: breakthrough experiments, modeling and simulation. Sep Purif Techn 90:246–256. https://doi.org/10.1016/j.seppur.2012.02.034
Naidu H, Mathews AP (2021) Linear driving force analysis of adsorption dynamics in stratified fixed-bed adsorbers. Sep Purif Technol 257:117955. https://doi.org/10.1016/j.seppur.2020.117955
Zhao H, Zhao N, Wang Q, Li F, Wang F, Fan S, Matus EV, Ismagilov ZR, Li L, Xiao F (2021) Adsorption equilibrium and kinetics of CO2 on mesocellular foams modified HKUST-1: Experiment and simulation. J CO2 Util 44:101415. https://doi.org/10.1016/j.jcou.2020.101415
Adhikari AK, Lin K-S (2016) Improving CO2 adsorption capacities and CO2/N2 separation efficiencies of MOF-74(Ni, Co) by doping palladium-containing activated carbon. Chem Eng J 284:1348–1360. https://doi.org/10.1016/j.cej.2015.09.086
Chen H, Dong S, Zhang Y, He P (2022) A comparative study on energy efficient CO2 capture using amine grafted solid sorbent: Materials characterization, isotherms, kinetics and thermodynamics. Energy 239:122348. https://doi.org/10.1016/j.energy.2021.122348
Gaaloul N, Daouas N (2018) An extended approach of a Kalman smoothing technique applied to a transient nonlinear two -dimensional inverse heat conduction problem. Int J Ther Sci 134:224–241. https://doi.org/10.1016/j.ijthermalsci.2018.08.021
Fguiri A, Marvillet C, Jeday MR (2021) Estimation of fouling resistance in a phosphoric acid/steam heat exchanger using inverse method. Appl Ther Eng 192:116935. https://doi.org/10.1016/j.applthermaleng.2021.116935
Singh SK, Yadav MK, Sonawane R, Khandekar S, Muralidhar K (2017) Estimation of time-dependent wall heat flux from single thermocouple data. Int J Ther Sci 115:1–15. https://doi.org/10.1016/j.ijthermalsci.2017.01.010
Cuadrado DG, Marconnet A, Paniagua G (2020) Non-linear non-Iterative transient inverse conjugate heat transfer method applied to microelectronics. Int J Heat Mass Transf 152:119503. https://doi.org/10.1016/j.ijheatmasstransfer.2020.119503
Li S, Deng S, Zhao L, Zhao R, Lin M, Du Y, Lian Y (2020) Mathematical modeling and numerical investigation of carbon capture by adsorption: literature review and case study. Appl Energy 221:437–449. https://doi.org/10.1016/j.apenergy.2018.03.093
Feng C, Jiaqiang E, Han W, Deng Y, Zhang B, Zhao X, Han D (2021) Key technology and application analysis of zeolite adsorption for energy storage and heat-mass transfer process: a review. R Sustain Energy Rev 144:110954. https://doi.org/10.1016/j.rser.2021.110954
Silva JD, Oliveira CB (2012) Fluid dynamics modelling for a fixed bed gasifier using Laplace transform finite difference method. Proc Eng 42:753–769. https://doi.org/10.1016/j.proeng.2012.07.468
Korzeń A, Taler D (2015) Modeling of transient response of a plate fin and tube heat exchanger. Int J Ther Sci 92:188–198. https://doi.org/10.1016/j.ijthermalsci.2015.01.036
Cisek P, Taler D (2019) Numerical analysis and performance assessment of the thermal energy storage unit aimed to be utilized in smart electric thermal storage (SETS). Energy 173:755–771. https://doi.org/10.1016/j.energy.2019.02.096
Tahmasbi V, Noori S (2019) Application of Levenberg-Marquardt method for estimation of the thermophysical properties and thermal boundary conditions of decomposing materials. Heat Transf Eng 41:1–27. https://doi.org/10.1080/01457632.2018.1558010
Cui M, Yang K, Xu X-L, Wang S-D, Gao X-W (2016) A modified Levenberg–Marquardt algorithm for simultaneous estimation of multi-parameters of boundary heat flux by solving transient nonlinear inverse heat conduction problems. Int J Heat Mass Transf 97:908–916. https://doi.org/10.1016/j.ijheatmasstransfer.2016.02.085
Jribi S, Miyazaki T, Saha Bidyut B, Pal A, Younes Mohamed M, Koyama S, Maalej A (2017) Equilibrium and kinetics of CO2 adsorption onto activated carbon. Int J Heat Mass Trans 108:1941–1946. https://doi.org/10.1016/j.ijheatmasstransfer.2016.12.114
Majd M, Kordzadeh-Kermani V, Ghalandari V, Askari A, Sillanpää M (2022) Adsorption isotherm models: a comprehensive and systematic review (2010–2020). Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2021.151334
Armstrong MR, Shan B, Cheng Z, Wang D, Liu J, Mu B (2017) Adsorption and diffusion of carbon dioxide on the metal-organic framework CuBTB. Chem Eng Sci 167:10–17. https://doi.org/10.1016/j.ces.2017.03.049
Yin G, Liu Q, Zhenyu Liu WuW (2018) Extension of Kelvin equation to CO2 adsorption in activated carbon. Fuel Process Tech 174:118–122. https://doi.org/10.1016/j.fuproc.2018.02.006
Pighini C, Mijoin J, Magnoux P, Costentin G, Lauron-Pernot H (2011) Microcalorimetric and thermodynamic studies of CO2 and methanol adsorption on magnesium oxide. Appl Surf Sci 257:6952–6962. https://doi.org/10.1016/j.apsusc.2011.03.040
Cavenati S, Grande CA, Rodrigues AE (2004) Adsorption equilibrium of methane, carbon dioxide, and nitrogen on zeolite 13X at high pressures. J Chem Eng Data 49:1095–1101. https://doi.org/10.1021/je0498917
Ben Taher MA, El-Otmany H, El Rhafiki T, Kousksou T, Zeraouli Y (2021) Inverse method to describe crystallization of undercooled water in cold storage tank. J Energy Storage 36:102404. https://doi.org/10.1016/j.est.2021.102404
Wang H, An C, Duana M, Su J (2020) Transient thermal analysis of multilayer pipeline with phase change material. Appl Thermal Eng 165:114512. https://doi.org/10.1016/j.applthermaleng.2019.114512
Liu F-B (2011) A hybrid method for the inverse heat transfer of estimating fluid thermal conductivity and heat capacity. Int J Ther Sci 50:718–724. https://doi.org/10.1016/j.ijthermalsci.2010.11.020
Wu Y, Ren C, Li R, Yang X, Tu J, Jiang S (2018) Measurement on effective thermal diffusivity and conductivity of pebble bed under vacuum condition in high temperature gas-cooled reactor. Prog Nucl Energy 106:195–203. https://doi.org/10.1016/j.pnucene.2018.01.014
Ghazizadeh HR, Azimi A, Maerefat M (2012) An inverse problem to estimate relaxation parameter and order of fractionality in fractional single-phase-lag heat equation. Int J Heat Mass Transf 55:2095–2101. https://doi.org/10.1016/j.ijheatmasstransfer.2011.12.012
Ukrainczyk N (2009) Thermal diffusivity estimation using numerical inverse solution for 1D heat conduction. Int J Heat Mass Transf 52:5675–5681. https://doi.org/10.1016/j.ijheatmasstransfer.2009.07.029
Li G, Liu Z, Li J, Fang Y, Shan J, Guo S, Wang Z (2018) Modeling of ash agglomerating fluidized bed gasifier using back propagation neural network based on particle swarm optimization. Appl Therm Eng 129:1518–1526. https://doi.org/10.1016/j.applthermaleng.2017.10.134
Sajedi R, Faraji J, Kowsary F (2021) A new damping strategy of Levenberg-Marquardt algorithm with a fuzzy method for inverse heat transfer problem parameter estimation. Int C Heat Mass Transf 126:105433. https://doi.org/10.1016/j.icheatmasstransfer.2021.105433
Hao W (2021) A gradient descent method for solving a system of nonlinear equations. Appl Math Lett 112:106739. https://doi.org/10.1016/j.aml.2020.106739
Li K, Han X (2022) A distributed Gauss-Newton method for distribution system state estimation. Int J Electr Power Energy Systems 136:107694. https://doi.org/10.1016/j.ijepes.2021.107694
Wang M, Xu X, Yan Z, Wang H (2021) An online optimization method for extracting parameters of multi-parameter PV module model based on adaptive Levenberg-Marquardt algorithm. Energy Conv Manag 245:114611. https://doi.org/10.1016/j.enconman.2021.114611
Tourn BA, Hostos JCA, Fachinotti VD (2021) Implementation of total variation regularization-based approaches in the solution of linear inverse heat conduction problems concerning the estimation of surface heat fluxes. Int C Heat Mass Transf 125:105330. https://doi.org/10.1016/j.icheatmasstransfer.2021.105330
Oliveira AVS, Avrit A, Gradeck M (2022) Thermocouple response time estimation and temperature signal correction for an accurate heat flux calculation in inverse heat conduction problems. Int J Heat Mass Transf 185:122398. https://doi.org/10.1016/j.ijheatmasstransfer.2021.122398
Silva JD (2015) Numerical modelling for a catalytic trickle-bed reactor using Laplace transform technique. Chem Eng Trans 43:1573–1578. https://doi.org/10.3303/CET1543263
Silva JD (2011) Dynamic evaluation for liquid tracer in a trickle-bed reactor. J Braz Soc Mech Sci Eng 33:272–277. https://doi.org/10.1590/S1678-58782011000300002
Anjos EB, Silva JD (2019) Numerical simulation of the heat transfer for a three-phase reactor of fixed bed. IEEE Latin Am Transf 17:788–795. https://doi.org/10.1109/TLA.2019.8891947
Acknowledgements
The authors of this paper would like to thank the CNPq (National Council of Scientific and Technological Development) for the financial support given (Process 57354/2018).
Author information
Authors and Affiliations
Corresponding author
Additional information
Technical Editor: Francis HR Franca.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Silva, J.D. Inverse analysis applying the Levenberg–Marquardt method for simultaneously estimating parameters of the adsorption of CO2 on activated carbon in a fixed-bed adsorber. J Braz. Soc. Mech. Sci. Eng. 44, 460 (2022). https://doi.org/10.1007/s40430-022-03756-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40430-022-03756-9