Abstract
Purpose
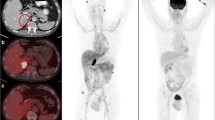
Contrast-enhanced computed tomography (CECT) underestimates the response to targeted therapy in metastatic renal cell cancer (mRCC) using Response Assessment Criteria In Solid Tumors (RECIST) v1.1, creating an unmet need for better imaging biomarkers for early response assessment. This study explores the feasibility of early response assessment to targeted therapy in metastatic clear cell RCC (ccRCC) using 68Ga-PSMA-11 PET/CT.
Methods
After informed consent, adult patients with biopsy-proven metastatic ccRCC planned for targeted therapy were recruited in this prospective observational pilot study from May 2021 to December 2022. 68Ga-PSMA-11 PET/CT was conducted at baseline and one and three months post-treatment. The concordance between PET and CT responses at one and three months was analysed. Follow-up and comparison of progression-free survival (PFS) between responders and non-responders on PET and CT was done.
Results
Twenty-one metastatic ccRCC patients were included in the final analysis. The response assessment on PET and CT was discordant in 15 out of 21 patients (~ 71%) at one month and in nine out of 21 patients (~ 43%) at three months. There was no significant difference between the median PFS of responders vs non-responders on CT at one (14 m vs. 12 m, p = 0.9) and three months (14 m vs. 12 m, p = 0.6). However, a higher difference in median PFS was observed between responders and non-responders on PET at one (PFS 14 m vs. 1 m, p < 0.001) and three months (PFS 14 m vs. 3 m, p = 0.28), respectively.
Conclusion
Early response assessment with 68Ga-PSMA-11 PET/CT is feasible and provides more accurate prognostic information than CECT in metastatic clear cell RCC patients undergoing targeted therapy.
Trial registration number
CTRI/2021/05/033805 (date of registration: 25/05/21)





Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Rock EP, Goodman V, Jiang JX, Mahjoob K, Verbois SL, Morse D, Dagher R, Justice R, Pazdur R (2007) Food and drug administration drug approval summary: sunitinib malate for the treatment of gastrointestinal stromal tumor and advanced renal cell carcinoma. Oncologist 12:107–113. https://doi.org/10.1634/theoncologist.12-1-107
Vasudev NS, Larkin JMG (2011) Tyrosine kinase inhibitors in the treatment of advanced renal cell carcinoma: focus on pazopanib. Clin Med Insights Oncol 5:333–342
Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S, Fernández-Pello S, Giles RH, Hofmann F, Hora M, Klatte T, Kuusk T, Lam TB, Marconi L, Powles T, Tahbaz R, Volpe A, Bex A (2022) European association of urology guidelines on renal cell carcinoma: the 2022 update. Eur Urol 82:399–410. https://doi.org/10.1016/j.eururo.2022.03.006
Motzer RJ, Jonasch E, Agarwal N, Alva A, Baine M, Beckermann K, Carlo MI, Choueiri TK, Costello BA, Derweesh IH, Desai A, Ged Y, George S, Gore JL, Haas N, Hancock SL, Kapur P, Kyriakopoulos C, Lam ET, Lara PN, Lau C, Lewis B, Madoff DC, Manley B, Dror Michaelson M, Mortazavi A, Nandagopal L, Plimack ER, Ponsky L, Ramalingam S, Shuch B, Smith ZL, Sosman J, Dwyer MA, Gurski LA, Motter A (2022) Kidney cancer, version 3.2022, nccn clinical practice guidelines in oncology. JNCCN J Nat Compr Cancer Netw 20:71–90. https://doi.org/10.6004/JNCCN.2022.0001
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/J.EJCA.2008.10.026
Serkova NJ, Eckhardt SG (2016) Metabolic imaging to assess treatment response to cytotoxic and cytostatic agents. Front Oncol 6:152. https://doi.org/10.3389/fonc.2016.00152
Tirumani SH, Fairchild A, Krajewski KM, Nishino M, Howard SA, Baheti AD, Rosenthal MH, Jagannathan JP, Shinagare AB, Ramaiya NH (2015) Weanti-VEGF molecular targeted therapies in common solid malignancies: comprehensive update for radiologists. Radiographics 35:455–474. https://doi.org/10.1148/rg.352140119
Smith AD, Lieber ML, Shah SN (2010) Assessing tumor response and detecting recurrence in metastatic renal cell carcinoma on targeted therapy: importance of size and attenuation on contrast-enhanced CT. Am J Roentgenol 194:157–165. https://doi.org/10.2214/AJR.09.2941
Smith AD, Shah SN, Rini BI, Lieber ML, Remer EM (2010) Morphology, attenuation, size, and structure (MASS) criteria: assessing response and predicting clinical outcome in metastatic renal cell carcinoma on antiangiogenic targeted therapy. Am J Roentgenol 194:1470–1478. https://doi.org/10.2214/AJR.09.3456
Shinagare AB, Krajewski KM, Braschi-Amirfarzan M, Ramaiya NH (2017) Advanced renal cell carcinoma: role of the radiologist in the era of precision medicine. Radiology 284:333–351
Chen R, Zhou X, Huang G, Liu J (2019) Fructose 1,6-bisphosphatase 1 expression reduces 18F-FDG uptake in clear cell renal cell carcinoma. Contrast Media Mol Imaging. https://doi.org/10.1155/2019/9463926
Jadvar H, Kherbache HM, Pinski JK, Conti PS (2003) Diagnostic role of [F-18]-FDG positron emission tomography in restaging renal cell carcinoma. Clin Nephrol 60:395–400. https://doi.org/10.5414/cnp60395
Caldarella C, Muoio B, Isgrò MA, Porfiri E, Treglia G, Giovanella L (2014) The role of fluorine 18 fluorodeoxyglucose positron emission tomography in evaluating the response to tyrosine-kinase inhibitors in patients with metastatic primary renal cell carcinoma. Radiol Oncol 48:219–227
Urso L, Bauckneht M, Albano D, Chondrogiannis S, Grassetto G, Lanfranchi F, Dondi F, Fornarini G, Lazzeri M, Evangelista L (2024) The evolution of PET imaging in renal, bladder, upper urinary tract urothelial, testicular and penile carcinoma – Today’s impact, tomorrow’s potential. Expert Rev Med Devices. https://doi.org/10.1080/17434440.2023.2293919
Chang SS, Reuter VE, Heston WDW, Bander NH, Grauer LS, Gaudin PB (1999) Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res 59:3192–3198
Spatz S, Tolkach Y, Jung K, Stephan C, Busch J, Ralla B, Rabien A, Feldmann G, Brossart P, Bundschuh RA, Ahmadzadehfar H, Essler M, Toma M, Müller SC, Ellinger J, Hauser S, Kristiansen G (2018) Comprehensive evaluation of prostate specific membrane antigen expression in the vasculature of renal tumors: implications for imaging studies and prognostic role. J Urol 199:370–377. https://doi.org/10.1016/j.juro.2017.08.079
Rhee H, Blazak J, Tham CM, Ng KL, Shepherd B, Lawson M, Preston J, Vela I, Thomas P, Wood S (2016) Pilot study: use of gallium-68 PSMA PET for detection of metastatic lesions in patients with renal tumour. EJNMMI Res 6:76. https://doi.org/10.1186/s13550-016-0231-6
Siva S, Callahan J, Pryor D, Martin J, Lawrentschuk N, Hofman MS (2017) Utility of 68Ga prostate specific membrane antigen—positron emission tomography in diagnosis and response assessment of recurrent renal cell carcinoma. J Med Imaging Radiat Oncol 61:372–378. https://doi.org/10.1111/1754-9485.12590
Udovicich C, Callahan J, Bressel M, Ong WL, Perera M, Tran B, Azad A, Haran S, Moon D, Chander S, Shaw M, Eapen R, Goad J, Lawrentschuk N, Murphy DG, Hofman M, Siva S (2022) Impact of prostate-specific membrane antigen positron emission tomography/computed tomography in the management of oligometastatic renal cell carcinoma. Eur Urol Open Sci 44:60. https://doi.org/10.1016/J.EUROS.2022.08.001
Urso L, Castello A, Rocca GC, Lancia F, Panareo S, Cittanti C, Uccelli L, Florimonte L, Castellani M, Ippolito C, Frassoldati A, Bartolomei M (2022) Role of PSMA-ligands imaging in renal cell carcinoma management: current status and future perspectives. J Cancer Res Clin Oncol 148:1299–1311. https://doi.org/10.1007/S00432-022-03958-7
Conway RE, Joiner K, Patterson A, Bourgeois D, Rampp R, Hannah BC, McReynolds S, Elder JM, Gilfilen H, Shapiro LH (2013) Prostate specific membrane antigen produces pro-angiogenic laminin peptides downstream of matrix metalloprotease-2. Angiogenesis 16:847–860. https://doi.org/10.1007/s10456-013-9360-y
Mittlmeier LM, Unterrainer M, Rodler S, Todica A, Albert NL, Burgard C, Cyran CC, Kunz WG, Ricke J, Bartenstein P, Stief CG, Ilhan H, Staehler M (2021) 18F-PSMA-1007 PET/CT for response assessment in patients with metastatic renal cell carcinoma undergoing tyrosine kinase or checkpoint inhibitor therapy: preliminary results. Eur J Nucl Med Mol Imaging 48:2031–2037. https://doi.org/10.1007/s00259-020-05165-3
Seitz AK, Rauscher I, Haller B, Krönke M, Luther S, Heck MM, Horn T, Gschwend JE, Schwaiger M, Eiber M, Maurer T (2018) Preliminary results on response assessment using 68Ga-HBED-CC-PSMA PET/CT in patients with metastatic prostate cancer undergoing docetaxel chemotherapy. Eur J Nucl Med Mol Imaging 45:602–612. https://doi.org/10.1007/s00259-017-3887-x
Tatar G, Alçın G, Samancı NŞ, Fenercioglu ÖE, Beyhan E, Çermik TF (2022) Role of 18F-FDG PET/CT in the assessment of therapy response and clinical outcome in metastatic renal cell carcinoma treated with tyrosine kinase inhibitors or immunotherapy. Nucl Med Commun 43:701–709. https://doi.org/10.1097/MNM.0000000000001553
Nakaigawa N, Kondo K, Ueno D, Namura K, Makiyama K, Kobayashi K, Shioi K, Ikeda I, Kishida T, Kaneta T, Minamimoto R, Tateishi U, Inoue T, Yao M (2017) The acceleration of glucose accumulation in renal cell carcinoma assessed by FDG PET/CT demonstrated acquisition of resistance to tyrosine kinase inhibitor therapy. BMC Cancer. https://doi.org/10.1186/S12885-016-3044-0
Tariq A, Kwok M, Pearce A, Rhee H, Kyle S, Marsh P, Raveenthiran S, Wong D, McBean R, Westera J, Dunglison N, Esler R, Navaratnam A, Yaxley JW, Thomas P, Pattison DA, Roberts MJ (2022) The role of dual tracer PSMA and FDG PET/CT in renal cell carcinoma (RCC) compared to conventional imaging: a multi-institutional case series with intra-individual comparison. Urol Oncol: Seminars Orig Investig 40:66.e1-66.e9. https://doi.org/10.1016/j.urolonc.2021.11.006
Demirci E, Ocak M, Kabasakal L, Decristoforo C, Talat Z, Halaç M, Kanmaz B (2014) 68Ga-PSMA PET/CT imaging of metastatic clear cell renal cell carcinoma. Eur J Nucl Med Mol Imaging 41:1461–1462. https://doi.org/10.1007/s00259-014-2766-y
Liu Y, Wang G, Yu H, Wu Y, Lin M, Gao J, Xu B (2020) Comparison of 18F-DCFPyL and 18F-FDG PET/computed tomography for the restaging of clear cell renal cell carcinoma: Preliminary results of 15 patients. Nucl Med Commun 1299–1305. https://doi.org/10.1097/MNM.0000000000001285
Gao J, Meng L, Xu Q, Zhao X, Deng Y, Fu Y, Guo S, He K, Shi J, Wang F, Zhang S, Guo H (2022) 68Ga-PSMA-11 PET/CT parameter correlates with pathological VEGFR-2/PDGFR-β expression in renal cell carcinoma patients. Mol Imaging Biol 24:759–768. https://doi.org/10.1007/s11307-022-01725-1
Aggarwal P, Singh H, Das CK, Mavuduru RS, Kakkar N, Lal A, Gorsi U, Kumar R, Mittal BR (2024) Potential role of 68Ga-PSMA PET/CT in metastatic renal cell cancer: a prospective study. Eur J Radiol 170:111218. https://doi.org/10.1016/J.EJRAD.2023.111218
Sagie S, Sarfaty M, Levartovsky M, Sorotsky HG, Berger R, Percik R, Gadot M (2022) RCC real-world data: prognostic factors and risk stratification in the immunotherapy era. Cancers (Basel). https://doi.org/10.3390/CANCERS14133127
Kryza D, Vinceneux A, Bidaux AS, Garin G, Tatu D, Cropet C, Badel JN, Perol D, Giraudet AL (2024) A multicentric, single arm, open-label, phase I/II study evaluating PSMA targeted radionuclide therapy in adult patients with metastatic clear cell renal cancer (PRadR). BMC Cancer 24:1–8. https://doi.org/10.1186/S12885-023-11702-8/TABLES/1
Acknowledgements
We thank the Indian Council of Medical Research (ICMR), New Delhi, India, for providing a research grant for the study (ref ID: MD21JUN-0007). We thank Dr Swayamjeet Satapathy, Senior resident, Department of Nuclear Medicine, All India Institute of Medical Sciences, New Delhi, for his kind assistance in the statistical analysis.
Funding
This work was supported by the Indian Council of Medical Research (ICMR), New Delhi, India (research grant ref ID: MD21JUN-0007).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study's conception and design. PA, HS, CKD and RSM were involved in patient selection, Material preparation, data collection and analysis were performed by PA, HS, RK, UG, AL and PP. The histopathological data was reviewed by NK. The first draft of the manuscript was written by PA and HS and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Post Graduate Institute of Medical Education and Research, Chandigarh (Date 11.05.21)(NK/7242/MD/040).
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aggarwal, P., Singh, H., Das, C.K. et al. Early response assessment to targeted therapy in metastatic clear cell renal cancer using 68Ga-PSMA-11 PET/CT and comparison with CECT: a feasibility study. Clin Transl Imaging (2024). https://doi.org/10.1007/s40336-024-00627-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40336-024-00627-2