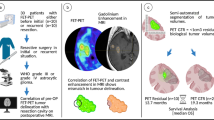
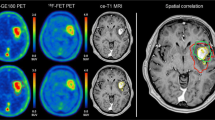
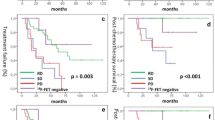
Abstract
Purpose
The possibility to provide answers from morphological imaging to neurosurgeon needs in patients with high- and low-grade gliomas relies on FLAIR images and percentage increase of contrast enhancement on T1-weighted images. Molecular imaging investigating the functionality of tumor cells has been progressively introduced in surgery planning to overcome limitations of conventional MRI. The purpose is to highlight the potential diagnostic and prognostic role of 18F-FET PET in gliomas.
Methods
We performed a literature review for articles on the topic in PubMed, Google Scholar, and Web of Science until January 2022. Search keywords included “glioma”, “glioblastoma”, “tumor biology” “18F-FET”, “MRI”, “surgery”.
Results
This review provides evidence of the potential of molecular imaging with 18F-FET to address the surgeon diagnostic and prognostic needs, including the knowledge of precise burden of cancer to guide biopsy or maximal safe resection, tumor grade and residual disease. The main cornerstones underlying the capacity of molecular imaging to provide information concerning tumor biology are deeply discussed to make the reader confident with the role of 18F-FET as a reliable imaging bio-marker in gliomas.
Conclusion
Although conventional MRI has been shown to be reliable technique to identify gliomas in terms of morphological concerns, some important limitations depending on the incapacity to reveal the tumor biology have emerged. The 18F-FET PET has been established to provide early information of functional nature including real tumor extension, grade, and metabolic residual disease. Therefore, the use of 18F-FET PET has progressively increased suggesting a potential shift from a morphological to functional plus morphological neurosurgery.




Similar content being viewed by others
References
Ostrom QT, Bauchet L, Davis FG et al (2014) The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol 16(7):896–913. https://doi.org/10.1093/neuonc/nou087
Braunstein S, Raleigh D, Bindra R, Mueller S, Haas-Kogan D (2017) Pediatric high-grade glioma: current molecular landscape and therapeutic approaches. J Neurooncol. 134(3):541–549
Minturn JE, Fisher MJ (2013) Gliomas in Children. Curr Treat Options Neurol 15(3):316–327. https://doi.org/10.1007/s11940-013-0225-x
Xu S, Tang L, Li X, Fan F, Liu Z (2020) Immunotherapy for glioma: current management and future application. Cancer Lett. 476:1–12
Bush NAO, Chang SM, Berger MS (2017) Current and future strategies for treatment of glioma. Neurosurg Rev 40(1):1–14. https://doi.org/10.1007/s10143-016-0709-8
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996. https://doi.org/10.1056/NEJMoa043330
Henriksson R, Asklund T, Poulsen HS (2011) Impact of therapy on quality of life, neurocognitive function and their correlates in glioblastoma multiforme: a review. J Neurooncol 104(3):639–646. https://doi.org/10.1007/s11060-011-0565-x
Korja M, Raj R, Seppä K et al (2019) Glioblastoma survival is improving despite increasing incidence rates: a nationwide study between 2000 and 2013 in Finland. Neuro Oncol 21(3):370–379. https://doi.org/10.1093/neuonc/noy164
Lattanzi W, Ripoli C, Greco V et al (2021) Basic and preclinical research for personalized medicine. J Pers Med 11(5):354. https://doi.org/10.3390/jpm11050354
Ma R, Taphoorn MJB, Plaha P (2021) Advances in the management of glioblastoma. J Neurol Neurosurg Psychiatry 92(10):1103–1111. https://doi.org/10.1136/jnnp-2020-325334
Galldiks N, Langen KJ, Pope WB (2015) From the clinician’s point of view - what is the status quo of positron emission tomography in patients with brain tumors? Neuro Oncol 17(11):1434–1444. https://doi.org/10.1093/neuonc/nov118
Molinaro AM, Hervey-Jumper S, Morshed RA, Young J, Han SJ, Chunduru P, Zhang Y, Phillips JJ, Shai A, Lafontaine M, Crane J, Chandra A, Flanigan P, Jahangiri A, Cioffi G, Ostrom Q, Anderson JE, Badve C, Barnholtz-Sloan J, Sloan AE, Erickson BJ, Decker PAB (2020) Association of maximal extent of resection of contrast-enhanced and non-contrast-enhanced tumor with survival within molecular subgroups of patients with newly diagnosed glioblastoma. JAMA Oncol 6(4):495–503. https://doi.org/10.1001/jamaoncol.2019.6143
Stupp R, Dietrich P-Y, Kraljevic SO et al (2002) Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol 20(5):1375–1382. https://doi.org/10.1200/JCO.2002.20.5.1375
Comeau ZJ, Lessard BH, Shuhendler AJ (2022) The need to pair molecular monitoring devices with molecular imaging to personalize health. Mol Imaging Biol. https://doi.org/10.1007/s11307-022-01714-4
Zaccagna F, Grist JT, Quartuccio N et al (2021) Imaging and treatment of brain tumors through molecular targeting: recent clinical advances. Eur J Radiol 142:109842. https://doi.org/10.1016/j.ejrad.2021.109842
Chukwueke UN, Wen PY (2019) Use of the response assessment in neuro-oncology (RANO) criteria in clinical trials and clinical practice. CNS Oncol. 8(1):CNS28. https://doi.org/10.2217/cns-2018-0007
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28(11):1963–1972. https://doi.org/10.1200/JCO.2009.26.3541
Zhang J, Liu H, Tong H et al (2017) Clinical applications of contrast-enhanced perfusion MRI techniques in gliomas: recent advances and current challenges. Contrast Media Mol Imaging 2017:1–27. https://doi.org/10.1155/2017/7064120
Albert NL, Weller M, Suchorska B et al (2016) Response assessment in neuro-oncology working group and european association for neuro-oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol 18(9):1199–1208. https://doi.org/10.1093/neuonc/now058
Wen PY, Chang SM, Van den Bent MJ, Vogelbaum MA, Macdonald DR, Lee EQ (2017) Response assessment in neuro-oncology clinical trials. J Clin Oncol 35(21):2439–2449. https://doi.org/10.1200/JCO.2017.72.7511
Law I, Albert NL, Arbizu J et al (2019) Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: version 1.0. Eur J Nucl Med Mol Imaging. 46(3):540–557. https://doi.org/10.1007/s00259-018-4207-9
Louis DN, Perry A, Wesseling P et al (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23(8):1231–1251. https://doi.org/10.1093/neuonc/noab106
Miyake K, Ogawa D, Okada M, Hatakeyama T, Tamiya T (2016) Usefulness of positron emission tomographic studies for gliomas. Neurol Med Chir (Tokyo) 56(7):396–408. https://doi.org/10.2176/nmc.ra.2015-0305
Alam IS, Arshad MA, Nguyen Q-D, Aboagye EO (2015) Radiopharmaceuticals as probes to characterize tumour tissue. Eur J Nucl Med Mol Imaging 42(4):537–561. https://doi.org/10.1007/s00259-014-2984-3
Muthu M, Nordström A (2019) Current status and future prospects of clinically exploiting cancer-specific metabolism-why is tumor metabolism not more extensively translated into clinical targets and biomarkers? Int J Mol Sci 20(6):1385. https://doi.org/10.3390/ijms20061385
Pavlova NN, Thompson CB (2016) The emerging hallmarks of cancer metabolism. Cell Metab 23(1):27–47. https://doi.org/10.1016/j.cmet.2015.12.006
Venneti S, Dunphy MP, Zhang H et al (2015) Glutamine-based PET imaging facilitates enhanced metabolic evaluation of gliomas in vivo. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aaa1009
Ju H-Q, Lin J-F, Tian T, Xie D, Xu R-H (2020) NADPH homeostasis in cancer: functions, mechanisms and therapeutic implications. Signal Transduct Target Ther 5(1):231. https://doi.org/10.1038/s41392-020-00326-0
Zhang J, Pavlova NN, Thompson CB (2017) Cancer cell metabolism: the essential role of the nonessential amino acid, glutamine. EMBO J 36(10):1302–1315. https://doi.org/10.15252/embj.201696151
Lin L, Yee SW, Kim RB, Giacomini KM (2015) SLC transporters as therapeutic targets: emerging opportunities. Nat Rev Drug Discov 14(8):543–560. https://doi.org/10.1038/nrd4626
Lopes C, Pereira C, Medeiros R (2021) ASCT2 and LAT1 contribution to the hallmarks of cancer: from a molecular perspective to clinical translation. Cancers (Basel) 13(2):203. https://doi.org/10.3390/cancers13020203
Fotiadis D, Kanai Y, Palacín M (2013) The SLC3 and SLC7 families of amino acid transporters. Mol Aspects Med 34(2–3):139–158. https://doi.org/10.1016/j.mam.2012.10.007
Scalise M, Pochini L, Console L, Losso MA, Indiveri C (2018) The human SLC1A5 (ASCT2) amino acid transporter: from function to structure and role in cell biology. Front Cell Dev Biol. https://doi.org/10.3389/fcell.2018.00096
Nałęcz KA (2020) Amino acid transporter SLC6A14 (ATB0,+) – a target in combined anti-cancer therapy. Front Cell Dev Biol. https://doi.org/10.3389/fcell.2020.594464
Kovalchuk V, Samluk Ł, Juraszek B et al (2019) Trafficking of the amino acid transporter B0,+ (SLC6A14) to the plasma membrane involves an exclusive interaction with SEC24C for its exit from the endoplasmic reticulum. Biochim Biophys Acta Mol Cell Res 1866(2):252–263. https://doi.org/10.1016/j.bbamcr.2018.11.005
Daubner SC, Le T, Wang S (2011) Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys 508(1):1–12. https://doi.org/10.1016/j.abb.2010.12.017
Saxton RA, Sabatini DM (2017) mTOR signaling in growth, metabolism, and disease. Cell 168(6):960–976. https://doi.org/10.1016/j.cell.2017.02.004
McCracken AN, Edinger AL (2013) Nutrient transporters: the Achilles’ heel of anabolism. Trends Endocrinol Metab 24(4):200–208. https://doi.org/10.1016/j.tem.2013.01.002
Yoshida GJ (2021) The harmonious interplay of amino acid and monocarboxylate transporters induces the robustness of cancer cells. Metabolites 11(1):27. https://doi.org/10.3390/metabo11010027
Lewerenz J, Hewett SJ, Huang Y et al (2013) The cystine/glutamate antiporter system x c − in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid Redox Signal 18(5):522–555. https://doi.org/10.1089/ars.2011.4391
Sontheimer H (2008) A role for glutamate in growth and invasion of primary brain tumors. J Neurochem 105(2):287–295. https://doi.org/10.1111/j.1471-4159.2008.05301.x
Kan LK, Drummond K, Hunn M, Williams D, O’Brien TJ, Monif M (2020) Potential biomarkers and challenges in glioma diagnosis, therapy and prognosis. BMJ Neurol Open 2(2):e000069. https://doi.org/10.1136/bmjno-2020-000069
Kristensen BW, Priesterbach-Ackley LP, Petersen JK, Wesseling P (2019) Molecular pathology of tumors of the central nervous system. Ann Oncol 30(8):1265–1278. https://doi.org/10.1093/annonc/mdz164
Bailey P, Cushing H (1925) Microchemical color reactions as an aid to the identification and classification of brain tumors. Proc Natl Acad Sci 11(1):82–84. https://doi.org/10.1073/pnas.11.1.82
Dunbar E, Yachnis AT (2010) Glioma diagnosis: immunohistochemistry and beyond. Adv Anat Pathol 17(3):187–201. https://doi.org/10.1097/PAP.0b013e3181d98cd9
Jaiswal S (2016) Role of immunohistochemistry in the diagnosis of central nervous system tumors. Neurol India 64(3):502. https://doi.org/10.4103/0028-3886.181547
Masui K, Cloughesy TF, Mischel PS (2012) Molecular pathology in adult high-grade gliomas: from molecular diagnostics to target therapies. Neuropathol Appl Neurobiol 38(3):271–291. https://doi.org/10.1111/j.1365-2990.2011.01238.x
Li L, Wang Y, Li Y, Fang S, Jiang T (2020) Role of molecular biomarkers in glioma resection: a systematic review. Chin Neurosurg J 6(1):1–7. https://doi.org/10.1186/s41016-020-00198-x
Elazab A, Bai H, Abdulazeem YM et al (2017) Post-surgery glioma growth modeling from magnetic resonance images for patients with treatment. Sci Rep 7(1):1222. https://doi.org/10.1038/s41598-017-01189-2
Kapur S (2001) Neuroimaging and drug development: an algorithm for decision making. J Clin Pharmacol 41(S7):64S-71S
Wester H-J (2007) Nuclear imaging probes: from bench to bedside. Clin Cancer Res 13(12):3470–3481. https://doi.org/10.1158/1078-0432.CCR-07-0264
McColl J, Holmes A, Ford I (1994) Statistical methods in neuroimaging with particular application to emission tomography. Stat Methods Med Res 3(1):63–86. https://doi.org/10.1177/096228029400300105
Enderling H, Chaplain M (2014) Mathematical modeling of tumor growth and treatment. Curr Pharm Des 20(30):4934–4940. https://doi.org/10.2174/1381612819666131125150434
Taghizadeh H, Müllauer L, Furtner J et al (2019) Applied precision cancer medicine in neuro-oncology. Sci Rep 9(1):20139. https://doi.org/10.1038/s41598-019-56473-0
Patel V, Shah J (2021) The current and future aspects of glioblastoma: immunotherapy a new hope? Eur J Neurosci 54(3):5120–5142. https://doi.org/10.1111/ejn.15343
Smith JS, Chang EF, Lamborn KR et al (2008) Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol 26(8):1338–1345. https://doi.org/10.1200/JCO.2007.13.9337
Brown TJ, Brennan MC, Li M et al (2016) Association of the extent of resection with survival in glioblastoma. JAMA Oncol 2(11):1460. https://doi.org/10.1001/jamaoncol.2016.1373
D’Amico RS, Englander ZK, Canoll P, Bruce JN (2017) Extent of resection in glioma–a review of the cutting edge. World Neurosurg 103:538–549. https://doi.org/10.1016/j.wneu.2017.04.041
Li YM, Suki D, Hess K, Sawaya R (2016) The influence of maximum safe resection of glioblastoma on survival in 1229 patients: can we do better than gross-total resection? J Neurosurg 124(4):977–988. https://doi.org/10.3171/2015.5.JNS142087
Kim Y-J, Lee DJ, Park C-K, Kim IA (2019) Optimal extent of resection for glioblastoma according to site, extension, and size: a population-based study in the temozolomide era. Neurosurg Rev 42(4):937–950. https://doi.org/10.1007/s10143-018-01071-3
Belhawi SMK, Hoefnagels FWA, Baaijen JC et al (2011) Early postoperative MRI overestimates residual tumour after resection of gliomas with no or minimal enhancement. Eur Radiol 21(7):1526–1534. https://doi.org/10.1007/s00330-011-2081-y
Marner L, Henriksen OM, Lundemann M, Larsen VA, Law I (2017) Clinical PET/MRI in neurooncology: opportunities and challenges from a single-institution perspective. Clin Transl Imaging 5(2):135–149. https://doi.org/10.1007/s40336-016-0213-8
Chaichana KL, Jusue-Torres I, Navarro-Ramirez R et al (2014) Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol 16(1):113–122. https://doi.org/10.1093/neuonc/not137
Pessina F, Navarria P, Cozzi L et al (2017) Maximize surgical resection beyond contrast-enhancing boundaries in newly diagnosed glioblastoma multiforme: is it useful and safe? A single institution retrospective experience. J Neurooncol 135(1):129–139. https://doi.org/10.1007/s11060-017-2559-9
Bénard F, Romsa J, Hustinx R (2003) Imaging gliomas with positron emission tomography and single-photon emission computed tomography. Semin Nucl Med 33(2):148–162. https://doi.org/10.1053/snuc.2003.127304
Chen W (2007) Clinical applications of PET in brain tumors. J Nucl Med 48(9):1468–1481. https://doi.org/10.2967/jnumed.106.037689
Cuddapah VA, Robel S, Watkins S, Sontheimer H (2014) A neurocentric perspective on glioma invasion. Nat Rev Neurosci 15(7):455–465. https://doi.org/10.1038/nrn3765
Jain R, Gutierrez J, Narang J et al (2011) In vivo correlation of tumor blood volume and permeability with histologic and molecular angiogenic markers in gliomas. Am J Neuroradiol 32(2):388–394. https://doi.org/10.3174/ajnr.A2280
Eidel O, Burth S, Neumann J-O et al (2017) Tumor infiltration in enhancing and non-enhancing parts of glioblastoma: a correlation with histopathology. PLoS ONE 12(1):e0169292. https://doi.org/10.1371/journal.pone.0169292
Lasocki A, Gaillard F (2019) Non-contrast-enhancing tumor: a new frontier in glioblastoma research. Am J Neuroradiol 40(5):758–765. https://doi.org/10.3174/ajnr.A6025
Sharma M, Juthani RG, Vogelbaum MA (2017) Updated response assessment criteria for high-grade glioma: beyond the MacDonald criteria. Chin Clin Oncol 6(4):37–50. https://doi.org/10.21037/cco.2017.06.26
Song S, Cheng Y, Ma J et al (2020) Simultaneous FET-PET and contrast-enhanced MRI based on hybrid PET/MR improves delineation of tumor spatial biodistribution in gliomas: a biopsy validation study. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-019-04656-2
Huang RY, Wen PY (2020) Indications and limitations of conventional imaging current clinical practice in the context of standard therapy. In: Pope W (ed) Glioma imaging. Springer, Cham, pp 1–15. https://doi.org/10.1007/978-3-030-27359-0_1
Lemée J-M, Clavreul A, Menei P (2015) Intratumoral heterogeneity in glioblastoma: don’t forget the peritumoral brain zone. Neuro Oncol 17(10):1322–1332. https://doi.org/10.1093/neuonc/nov119
Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V (2011) Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol 12(11):997–1003. https://doi.org/10.1016/S1470-2045(11)70196-6
Jenkinson MD, Barone DG, Bryant A, Vale L, Bulbeck H, Lawrie TA, Hart MG, Watts C (2018) Intraoperative imaging technology to maximize extent of resection for glioma. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD012788.pub2
Zhang ZZ, Shields LBE, Sun DA, Zhang YP, Hunt MA, Shields CB (2015) The art of intraoperative glioma identification. Front Oncol. https://doi.org/10.3389/fonc.2015.00175
Muragaki Y, Iseki H, Maruyama T, Kawamata T, Yamane F, Nakamura R, Kubo O, Takakura K, Hori T (2006) Usefulness of intraoperative magnetic resonance imaging for glioma surgery. Acta Neurochir Suppl 98:67–75. https://doi.org/10.1007/978-3-211-33303-7_10.79
Villanueva-Meyer JE, Mabray MC, Cha S (2017) Current clinical brain tumor imaging. Neurosurgery 81(3):397–415. https://doi.org/10.1093/neuros/nyx103
Verburg N, de Witt Hamer PC (2021) State-of-the-art imaging for glioma surgery. Neurosurg Rev 44(3):1331–1343. https://doi.org/10.1007/s10143-020-01337-9
Forte E, Fiorenza D, Torino E et al (2019) Radiolabeled PET/MRI nanoparticles for tumor imaging. J Clin Med. https://doi.org/10.3390/jcm9010089
Zhang-Yin JT, Girard A, Bertaux M (2022) What does PET imaging bring to neuro-oncology in 2022? A review. Cancers (Basel) 14(4):879. https://doi.org/10.3390/cancers14040879
Wester HJ, Herz M, Weber W et al (1999) Synthesis and radiopharmacology of O-(2-[18F]fluoroethyl)-L-tyrosine for tumor imaging. J Nucl Med. 40(1):205–212
Zuhayra M, Alfteimi A, Von FC, Lützen U, Meller B, Henze E (2009) New approach for the synthesis of [18F]fluoroethyltyrosine for cancer imaging: simple, fast, and high yielding automated synthesis. Bioorg Med Chem 17(21):7441–7448. https://doi.org/10.1016/j.bmc.2009.09.029
Lisova K, Chen BY, Wang J, Fong KM-M, Clark PM, van Dam RM (2020) Rapid, efficient, and economical synthesis of PET tracers in a droplet microreactor: application to O-(2-[18F]fluoroethyl)-L-tyrosine ([18F]FET). EJNMMI Radiopharm Chem. 5(1):1. https://doi.org/10.1186/s41181-019-0082-3
Bourdier T, Greguric I, Roselt P, Jackson T, Faragalla J, Katsifis A (2011) Fully automated one-pot radiosynthesis of O-(2-[18F]fluoroethyl)-l-tyrosine on the tracerlab FXFN module. Nucl Med Biol 38(5):645–651. https://doi.org/10.1016/j.nucmedbio.2011.01.001
Chao M, Chezal J-M, Debiton E et al (2022) A convenient route to new (radio)fluorinated and (radio)iodinated cyclic tyrosine analogs. Pharmaceuticals 15(2):162. https://doi.org/10.3390/ph15020162
Gladson CL, Prayson RA, Liu WM (2010) The pathobiology of glioma tumors. Annu Rev Pathol Mech Dis 5(1):33–50. https://doi.org/10.1146/annurev-pathol-121808-102109
Vettermann FJ, Diekmann C, Weidner L et al (2021) L-type amino acid transporter (LAT) 1 expression in 18F-FET-negative gliomas. EJNMMI Res 11(1):124. https://doi.org/10.1186/s13550-021-00865-9
Zhang J, Xu Y, Li D et al (2020) Review of the correlation of LAT1 with diseases: mechanism and treatment. Front Chem. https://doi.org/10.3389/fchem.2020.564809
Galldiks N, Lohmann P, Albert NL, Tonn JC, Langen K-J (2019) Current status of PET imaging in neuro-oncology. Neuro-Oncol Adv. https://doi.org/10.1093/noajnl/vdz010
Arvanitis CD, Ferraro GB, Jain RK (2020) The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat Rev Cancer 20(1):26–41. https://doi.org/10.1038/s41568-019-0205-x
Fuenfgeld B, Mächler P, Fischer DR et al (2020) Reference values of physiological 18F-FET uptake: implications for brain tumor discrimination. PLoS ONE 15(4):1–16. https://doi.org/10.1371/journal.pone.0230618
Law I, Albert NL, Arbizu J et al (2019) Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18 F]FDG: version 1.0. Eur J Nucl Med Mol Imaging. 46(3):540–557. https://doi.org/10.1007/s00259-018-4207-9
Hua T, Zhou W, Zhou Z, Guan Y, Li M (2021) Heterogeneous parameters based on 18F-FET PET imaging can non-invasively predict tumor grade and isocitrate dehydrogenase gene 1 mutation in untreated gliomas. Quant Imaging Med Surg 11(1):317–327. https://doi.org/10.21037/QIMS-20-723
Hadjipanayis CG, Widhalm G, Stummer W (2015) What is the surgical benefit of utilizing 5-aminolevulinic acid for fluorescence-guided surgery of malignant gliomas? Neurosurgery 77(5):663–673. https://doi.org/10.1227/NEU.0000000000000929
Lau D, Hervey-Jumper SL, Chang S et al (2016) A prospective Phase II clinical trial of 5-aminolevulinic acid to assess the correlation of intraoperative fluorescence intensity and degree of histologic cellularity during resection of high-grade gliomas. J Neurosurg 124(5):1300–1309. https://doi.org/10.3171/2015.5.JNS1577
Golub D, Hyde J, Dogra S et al (2021) Intraoperative MRI versus 5-ALA in high-grade glioma resection: a network meta-analysis. J Neurosurg 134(2):484–498. https://doi.org/10.3171/2019.12.JNS191203
Schucht P, Knittel S, Slotboom J et al (2014) 5-ALA complete resections go beyond MR contrast enhancement: shift corrected volumetric analysis of the extent of resection in surgery for glioblastoma. Acta Neurochir (Wien) 156(2):305–312. https://doi.org/10.1007/s00701-013-1906-7
Celli M, Caroli P, Amadori E et al (2021) Diagnostic and prognostic potential of 18F-FET PET in the differential diagnosis of glioma recurrence and treatment-induced changes after chemoradiation therapy. Front Oncol 11:1–10. https://doi.org/10.3389/fonc.2021.721821
Dunet V, Rossier C, Buck A, Stupp R, Prior JO (2012) Performance of 18F-fluoro-ethyl-tyrosine (18F-FET) PET for the differential diagnosis of primary brain tumor: a systematic review and metaanalysis. J Nucl Med 53(2):207–214
Näslund O, Smits A, Förander P, Laesser M, Bartek J Jr, Gempt J, Liljegren A, Daxberg ELJA (2018) Amino acid tracers in PET imaging of diffuse low-grade gliomas: a systematic review of preoperative applications. Acta Neurochir (Wien) 160(7):1451–1460
Acknowledgements
We are grateful to Nuclear Medicine Staff of S. Stefano Hospital of Prato - Azienda USL Toscana Centro (physicians, technicians, nurses and administrative) for the fruitful collaboration, discussion and competence demonstrated in the implementation of 18F-FET PET in the clinical routine.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
All authors equally contributed to the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Laghai, I., Muscas, G., Tardelli, E. et al. The new era of bio-molecular imaging with O-(2-18F-fluoroethyl)-L-tyrosine (18F-FET) in neurosurgery of gliomas. Clin Transl Imaging 10, 553–565 (2022). https://doi.org/10.1007/s40336-022-00509-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-022-00509-5