Abstract
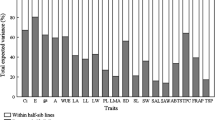
Intraspecific trait variation and heritability in different environmental conditions not only suggest a potential for an evolutionary response but also have important ecological consequences at the population, community, and ecosystem levels. However, the contribution of quantitative trait variation within a grassland species to evolutionary responses or ecological consequences is seldom documented. Leymus chinensis is an important dominant species in semi-arid grasslands of China, which has seriously suffered from drought and high temperature stresses in recent decades. In the present study, we measured variation and heritability of 10 quantitative traits, namely the number of tillers, maximum shoot height, number of rhizomes, maximum rhizome length, rhizome mass, aboveground mass, root mass, maximum net photosynthetic rate (Pmax), specific leaf area (SLA), and leaf length to leaf width ratio (LL/LW), for 10 genotypes of L. chinensis under one non-stress (Ck) condition and three environmental stress conditions (i.e., drought (Dr), high temperature (Ht), and both drought and high temperature (DrHt)). Result indicated that (1) the interaction of genotype and environmental condition (G×E) was significant for 6 traits but not significant for the other 4 traits as shown by two-way analysis of variance (ANOVA), suggesting that different selection forces were placed for different traits on the factors dominating phenotypic responses to different environmental conditions. Moreover, these significant G×E effects on traits indicated significantly different phenotypic adaptive responses among L. chinensis genotypes to different environmental conditions. Additionally, individuals could be grouped according to environmental condition rather than genotype as shown by canonical discriminant analysis, indicating that environmental condition played a more important role in affecting phenotypic variation than genotype; (2) by one-way ANOVA, significant differences among L. chinensis genotypes were found in all 10 traits under Ck and Dr conditions, in 8 traits under DrHt condition and only in 4 traits under Ht condition; and (3) all 10 traits showed relatively low or non-measurable broad-sense heritability (H2) under stress conditions. However, the lowest H2 value for most traits did not occur under DrHt condition, which supported the hypothesis of 'unfavorable conditions have unpredictable effects' rather than 'unfavorable conditions decrease heritability'. Results from our experiment might aid to improve predictions on the potential impacts of climate changes on L. chinensis and eventually species conservation and ecosystem restoration.
Similar content being viewed by others
References
Badyaev A V. 2005. Stress-induced variation in evolution: from behavioural plasticity to genetic assimilation. Proceedings of the Royal Society B: Biological Sciences, 272(1566): 877–886.
Bai Y F, Han X G, Wu J G, et al. 2004. Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature, 431(7005): 181–184.
Bischoff A, Steinger T, Müller-Schärer H. 2010. The importance of plant provenance and genotypic diversity of seed material used for ecological restoration. Restoration Ecology, 18(3): 338–348.
Bolnick D I, Amarasekare P, Araújo M S, et al. 2011. Why intraspecific trait variation matters in community ecology. Trends in Ecology & Evolution, 26(4): 183–192.
Charmantier A, Garant D. 2005. Environmental quality and evolutionary potential: lessons from wild populations. Proceedings of the Royal Society B: Biological Sciences, 272(1571): 1415–1425.
Cook-Patton S C, McArt S H, Parachnowitsch A L, et al. 2011. A direct comparison of the consequences of plant genotypic and species diversity on communities and ecosystem function. Ecology, 92(4): 915–923.
Cornelissen J H C, Lavorel S, Garnier E, et al. 2003. A handbook of protocols for standardized and easy measurement of plant functional traits worldwide. Australian Journal of Botany, 51(4): 335–380.
Crawford K M, Rudgers J A. 2013. Genetic diversity within a dominant plant outweighs plant species diversity in structuring an arthropod community. Ecology, 94(5): 1025–1035.
Crutsinger G M, Strauss S Y, Rudgers J A. 2010. Genetic variation within a dominant shrub species determines plant species colonization in a coastal dune ecosystem. Ecology, 91(4): 1237–1243.
Crutsinger G M. 2016. A community genetics perspective: opportunities for the coming decade. New Phytologist, 210(1): 65–70.
Dia M, Wehner T C, Hassell R, et al. 2016. Genotype×environment interaction and stability analysis for watermelon fruit yield in the United States. Crop Science, 56(4): 1645–1661.
Draghi J A, Whitlock M C. 2012. Phenotypic plasticity facilitates mutational variance, genetic variance, and evolvability along the major axis of environmental variation. Evolution, 66(9): 2891–2902.
Espeland E K, Emery N C, Mercer K L, et al. 2017. Evolution of plant materials for ecological restoration: insights from the applied and basic literature. Journal of Applied Ecology, 54(1): 102–115.
Ghalambor C K, McKay J K, Carroll S P, et al. 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Functional Ecology, 21(3): 394–407.
Gustafson D J, Major C, Jones D, et al. 2014. Genetic sorting of subordinate species in grassland modulated by intraspecific variation in dominant species. PloS ONE, 9(3): e91511, doi: 10.1371/journal.pone.0091511.
Hallsson L R, Björklund M. 2012. Selection in a fluctuating environment leads to decreased genetic variation and facilitates the evolution of phenotypic plasticity. Journal of Evolutionary Biology, 25(7): 1275–1290.
Hoffmann A A, Merilä J. 1999. Heritable variation and evolution under favourable and unfavourable conditions. Trends in Ecology & Evolution, 14 (3): 96–101.
Hughes A R, Inouye B D, Johnson M T, et al. 2008. Ecological consequences of genetic diversity. Ecology Letters, 11(6): 609–623.
Kraft N J B, Crutsinger G M, Forrestel E J, et al. 2014. Functional trait differences and the outcome of community assembly: an experimental test with vernal pool annual plants. Oikos, 123(11): 1391–1399.
Liu Z P, Li X F, Li H J, et al. 2007. The genetic diversity of perennial Leymus chinensis originating from China. Grass and Forage Science, 62 (1): 27–34.
Lynch M, Walsh B. 1998. Genetics and Analysis of Quantitative Traits. Sunderland: Sinauer Associates, Inc., 548–550.
Manaa A, Ahmed H B, Valot B, et al. 2011. Salt and genotype impact on plant physiology and root proteome variations in tomato. Journal of Experimental Botany, 62(8): 2797–2813.
Mittelbach G G, Osenberg C W, Wainwright P C. 1999. Variation in feeding morphology between pumpkinseed populations: Phenotypic plasticity or evolution? Evolutionary Ecology Research, 1(1): 111–128.
Morris G P, Hu Z B, Grabowski P P, et al. 2016. Genotypic diversity effects on biomass production in native perennial bioenergy cropping systems. Global Change Biology Bioenergy, 8(5): 1000–1014.
Nicotra A B, Atkin O K, Bonser S P, et al. 2010. Plant phenotypic plasticity in a changing climate. Trends in Plant Science, 15(12): 684–692.
Pigliucci M. 2005. Evolution of phenotypic plasticity: where are we going now? Trends in Ecology & Evolution, 20(9): 481–486.
Prieto I, Violle C, Barre P, et al. 2015. Complementary effects of species and genetic diversity on productivity and stability of sown grasslands. Nature Plants, 1(4): 15033.
Qiu H, Huang S, Zhang Y. 1996. Flora Peipublicae Popularis Sinicae, Tomus 42(2). Beijing: Science Press, 133–147. (in Chinese)
Salmela M J, Greenham K, Lou P, et al. 2016. Variation in circadian rhythms is maintained among and within populations in Boechera stricta. Plant, Cell & Environment, 39(6): 1293–1303.
Shaw E J, Kakuda Y, Rajcan I. 2016. Effect of genotype, environment, and genotype×environment interaction on tocopherol accumulation in soybean seed. Crop Science, 56(1): 40–50.
Shen J F, Ren H Q, Xin X J, et al. 2015. Leymus chinensis genotypic diversity increase the response of population to disturbance. Acta Ecologia Sinica, 35(23): 7682–7689. (in Chinese)
Smith M D, Hoffman A M, Avolio M L. 2016. Gene expression patterns of two dominant tallgrass prairie species differ in response to warming and altered precipitation. Scientific Reports, 6: 25522.
Treplin M, Pennings S C, Zimmer M. 2013. Decomposition of leaf litter in a U.S. saltmarsh is driven by dominant species, not species complementarity. Wetlands, 33(1): 83–89.
Wang Y S, Zhao L M, Wang H, et al. 2005. Molecular genetic variation in a clonal plant population of Leymus chinensis (Trin.) Tzvel. Journal of Integrative Plant Biology, 47(9): 1055–1064.
Wang Z W, Li L H, Han X G, et al. 2004. Do rhizome severing and shoot defoliation affect clonal growth of Leymus chinensis at ramet population level? Acta Oecologica, 26(3): 255–260.
Xu S X, Shu Q Y, Liu G S. 2006. Genetic relationship in ecotypes of Leymus chinensis revealed by polymorphism of amplified DNA fragment lengths. Russian Journal of Plant Physiology, 53(5): 678–683.
Xu Z Z, Zhou G S. 2006. Combined effects of water stress and high temperature on photosynthesis, nitrogen metabolism and lipid peroxidation of a perennial grass Leymus chinensis. Planta, 224(5): 1080–1090.
Yang X, Shen J F, Zhao N X, et al. 2017. Phenotypic plasticity and genetic differentiation of quantitative traits in genotypes of Leymus chinensis. Chinese Journal of Plant Ecology, 41(3): 359–368. (in Chinese)
Acknowledgements
This research was funded by the National Key Research and Development Program of China (2016YFC0500706), the National Natural Science Foundation of China (31570427, 31770505), and the China Scholarship Council (201606205032). We are grateful to Dr. Pella BRINKMAN and Dr. RUAN Weibin for helpful suggestions on improving the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, X., Li, J., Zhao, T. et al. Variation and heritability of morphological and physiological traits among Leymus chinensis genotypes under different environmental conditions. J. Arid Land 11, 66–74 (2019). https://doi.org/10.1007/s40333-018-0018-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40333-018-0018-x