Abstract
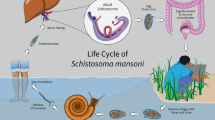
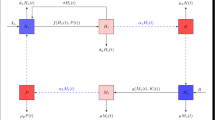
A system of nonlinear differential equations is proposed to assess the effects of prevalence-dependent disease contact rate, pathogen’s shedding rates, and treatment rate on the dynamics of schistosomiasis in a general setting. The decomposition techniques by Vidyasagar and the theory of monotone systems are the main ingredients to deal completely with the global asymptotic analysis of the system. Precisely, a threshold quantity for the analysis is derived and the existence of a unique endemic equilibrium is shown. Irrespective of the initial conditions, we prove that the solutions converge either to the disease-free equilibrium or to the endemic equilibrium, depending on whether the derived threshold quantity is less or greater than one. We assess the role of an integrated control strategy driven by human behavior changes through the incorporation of prevalence-dependent increasing the prophylactic treatment and decreasing the contact rate functions, as well as the mechanical water sanitation and the biological elimination of snails. Because schistosomiasis is endemic, the aim is to mitigate the endemic level of the disease. In this regard, we show both theoretically and numerically that: the reduction of contact rate through avoidance of contaminated water, the enhancement of prophylactic treatment, the water sanitation, and the removal of snails can reduce the endemic level and, to an ideal extent, drive schistosomiasis to elimination.













Similar content being viewed by others
References
Al F, Basir S, Ray E Venturino (2018) Role of media coverage and delay in controlling infectious diseases: a mathematical model. Appl Math Comput 337:372–385
Allan ERO, Tennessen JA, Bollmann SR, Hanington PC, Bayne CJ, Blouin MS (2017) Schistosome infectivity in the snail, Biomphalaria glabrata, is partially dependent on the expression of Grctm6, a guadeloupe resistance complex protein. PLoS Negl Trop Dis 11(2):e0005362
Anguelov R, Dumont Y, Lubuma J (2012) Mathematical modeling of sterile insect technology for control of anopheles mosquito. Comput Math Appl 64:374–389
Auger P, Kouokam E, Sallet G, Tchuente M, Tsanou B (2008) The Ross–Macdonald model in a patchy environment. Math Biosci 216:123–131
Bani-Yaghoub M, Gautam R, Shuai Z, van den Driessche P, Ivanek R (2012) Reproduction numbers for infections with free-living pathogens growing in the environment. J Biol Dynam 6:923–940
Barbour AD (1978) Macdonald’s model and the transmission of bilharzia. Trans R Soc Trop Med Hyg 72:6–15
Berman A, Plemmons RJ (1979) Nonnegative matrices in the mathematical sciences. Academic Press, New York
Bernot RJ (1999) Predator identity and consumer behavior: differential effects of fish and crayfish on the habitat use of a freshwater snail. Oecologia 118:242–247
Cai Y, Wang W (2017) Global stability for an influenza transmission model incorporating human mobility behavior. Int J Biomath 10:1750100
Caraco T, Wang IN (2008) Free-living pathogens: life-history constraints and strain competition. J Theor Biol 250:569–579
Chiyaka ET, Garira W (2009) Mathematical analysis of the transmission dynamics of schistosomiasis in the human-snail hosts. J Biol Syst 17:397–423
Chiyaka ET, Magombedze G, Mutimbu L (2010) Modelling within host parasite dynamics of schistosomiasis. Comput Math Methods Med 11:255–280
Castillo-Chavez C, Feng Z, Xu D (2008) A schistosomiasis model with mating structure and time delay. Math Biosci 211:333–341
Ding C, Qiu Z, Zhu H (2015) Multi-host transmission dynamics of schistosomiasis and its optimal control. Math Biosci Eng 12:983–1006
Ding C, Sun Y, Zhu Y (2017) A schistosomiasis compartment model with incubation and its optimal control. Math Methods Appl Sci 40:5079–5094
Djiomba Njankou SD, Nyabadza F (2017) Modelling the potential role of media campaigns in Ebola transmission dynamics. Int J Differ Equ (art. ID 3758269), p 13
Diaby M, Iggidr A, Sy M, Sène A (2014) Global analysis of a schistosomiasis infection model with biological control. Appl Math Comput 246:731–742
Diekmann O, Heesterbeek JAP, Metz JAJ (1990) On the definition and computation of the basic reproduction ratio \(R_0\) in models for infectious diseases in heterogeneous populations. J Math Biol 28:365–382
Diekmann O, Heesterbeek JAP, Roberts MG (2010) The construction of next-generation matrices for the compartmental epidemic models of disease transmission. J R Soc Interface 7:873–885
van den Driessche P, Watmough J (2002) Reproduction numbers and sub-threshold endemic equilibria for compartment models of disease transmission. Math Biosci 180:29–48
Feng Z, Eppert A, Milner FA, Minchella DJ (2004) Estimation of parameters governing the transmission dynamics of schistosomes. Appl Math Lett 17:1105–1112
Funk S, Bansal S, Bauch CT, Eames KTD, John Edmunds W, Galvani AP, Klepac P (2015) Nine challenges in incorporating the dynamics of behavior in infectious diseases models. Epidemics 10:21–25
Greenhalgh D, Rana S, Samanta S, Sardar T, Bhattacharya S, Chattopadhyay J (2015) Awareness programs control infectious disease multiple delay induced mathematical model. Appl Math Comput 251:539–563
Guiro A, Ngom D, Ouedraogo D (2017) Stability analysis for a class of discrete schistosomiasis models with general incidence. Adv Differ Equ 2017:116
Guiro A, Ouaro S, Traore A (2013) Stability analysis of a schistosomiasis model with delays. Adv Differ Equ 2013:303
Hethcote HW, Thieme HR (1985) Stability of the endemic equilibrium in epidemic models with subpopulations. Math Biosci 75:205–227
Hirsch MW (1988) Stability and convergence in strongly monotone dynamical systems. J Reine Angew Math 333:1–53
Hirsch MW (1985) Systems of differential equations that are competitive or cooperative II: convergence almost everywhere. SIAM J Math Anal 16:423–439
Iggidr A, Sallet G, Tsanou B (2012) Global stability analysis of a metapopulation SIS epidemic model. Math Popul Stud 19:115–129
Inobaya MT, Olveda RM, Chau TNP, Olveda DU, Ross AGP (2014) Prevention and control of schistosomiasis: a current perspective. Res Rep Trop Med 5:65–75
Ittiprasert W, Knight M (2012) Reversing the resistance phenotype of the Biomphalaria glabrata snail host Schistosoma mansoni infection by temperature modulation. PLoS Pathog 8(4):e1002677
Kiss IZ (2013) Incorporating human behavior in epidemic dynamics: a modelling perspective. In: Manfredi P, D’Onofrio A (eds) Modeling the interplay between human behavior and the spread of infectious diseases. Springer, New York, pp 125–137
Kloos H (1995) Human behavior, health education and schistosomiasis control: a review. Soc Sci Med 40:149–1511
LaSalle JP (1976) The stability of dynamical systems. In: CBMS-NSF regional conference series in applied mathematics. SIAM, Philadelphia, Pa
Li Q, Lu F, Dai C, Fan M, Wang W, Wang K (2017) Simulating the potential role of media coverage and infected bats in the crossmark 4 2014 Ebola outbreak. J Theor Biol 412(2017):123–129
Lin L, Zhicheng L (2014) Existence of periodic solutions and stability of zero solution of a mathematical model of schistosomiasis. J Appl Math (art. ID 765498), p 8
Long-xing Q, Jing-an C (2013) A schistosomiasis model with Praziquantel resistance. Discret Dyn Nat Soc (art. ID 945767), p 14
Long-xing Q, Jing-an C (2012) Qualitative analysis for Barbour’s schistosomiasis model with diffusion. J Biomath 27:54–64
Long-xing Q, Jing-An C (2012) The delayed Barbour’s model for schistosomiasis. Int J Biomath 5:1250024
Lu X, Wang S, Liu S, Li J (2017) An SEI infection model incorporating media impact. Math Biosci Eng 14:1317–1335
Macdonald G (1965) The dynamics of helminth infections, with special reference to schistosomes. Trans R Soc Trop Med Hyg 59:489–506
Manfredi P, D’Onofrio A (2013) Modeling the interplay between human behavior and the spread of infectious diseases. Springer, New York
Mangal TD, Paterson S, Fenton A (2008) Predicting the impact of long-term temperature changes on the epidemiology and control of schistosomiasis: a mechanistic model. PLoS One 3:e1438
Manyombe MM, Tsanou B, Mbang J, Bowong S (2017) A metapopulation model for the population dynamics of anopheles mosquito. Appl Math Comput 307:71–91
Mari L, Ciddio M, Casagrandi R, Perez-Saez J, Bertuzzo E, Rinaldo A, Sokolow SH, De Leo GA, Gatto M (2017) Heterogeneity in schistosomiasis transmission dynamics. J Theor Biol 432:87–99
Milner FA, Zhao R (2008) A deterministic model of schistosomiasis with spatial structure. Math Biosci Eng 5:505–522
Mushayabasa S, Bhunu CP (2011) Modeling schistosomiasis and HIV/AIDS co-dynamics. Comput Math Methods Med (art. ID 846174), p 15
Musuva RM, Awiti A, Omedo M, Ogutu M, Secor WE, Montgomery SP, Alaii J, Mwinzi PNM (2014) Community knowledge, attitudes and practices on schistosomiasis in Western Kenya—the SCORE project. Am J Trop Med Hyg 90:646–652
Nelson EJ, Harris JB, Morris JG, Calderwood SB, Camilli A (2009) Cholera transmission: the host, pathogen and bacteriophage dynamics. Nat Rev Microbiol 7:693–702
Ngarakana-Gwasira ET, Bhunu CP, Masocha M, Mashonjowa E (2016) Transmission dynamics of schistosomiasis in Zimbabwe: a mathematical and GIS approach. Commun Nonlinear Sci Numer Simul 35:137–147
Odhiambo GO, Musuva RM, Atuncha VO, Mutete ET, Odiere MR, Onyango RO, Alaii JA, Mwinzi PNM (2014) Low levels of awareness despite high prevalence of schistosomiasis among communities in Nyalenda informal settlement, Kisumu City, Western Kenya. PLoS Negl Trop Dis 8:e2784
Okosun KO, Smith R (2017) Optimal control analysis of malaria-schistosomiasis co-infection dynamics. Math Biosci Eng 14:377–405
Sahu GP, Dhar J (2015) Dynamics of an SEQIHRS epidemic model with media coverage, quarantine and isolation in a community with pre-existing immunity. J Math Anal Appl 421:1651–1672
Shan C, Gao H, Zhu H (2011) Dynamics of a delay Schistosomiasis model in snail infections. Math Biosci Eng 8:1099–1115
Shan C, Zhou X, Zhu H (2014) The dynamics of growing islets and transmission of schistosomiasis japonica in the Yangtze River. Bull Math Biol 76:1194–1217
Smith HL (1988) Systems of ordinary differential equations which generate an order preserving flow. A survey of results. SIAM Rev 30:87–113
Sokolowa SH, Huttinger E, Jouanard N, Hsiehd MH, Lafferty KD, Kuris AM, Riveau G, Senghor S, Thiam C, N’Diaye A, Faye DS, De Leo GA (2015) Reduced transmission of human schistosomiasis after restoration of a native river prawn that preys on the snail intermediate host. PNAS 112(31):9650–9655
Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J (2006) Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis 6:411–425
Vidyasagar M (1980) Decomposition techniques for large-scale systems with nonadditive interactions: stability and stabilization. IEEE Trans Autom Control 25:773–779
Sun C, Yang W, Arino J, Khan K (2011) Effect of media-induced social distancing on disease transmission in a two patch setting. Math Biosci 230:87–95
Wang X, Gao D, Wang J (2015) Influence of human behavior on cholera dynamics. Math Biosci 267:41–52
Wang X, Wang J (2015) Analysis of cholera epidemics with bacterial growth and spatial movement. J Biol Dyn 9:233–261
Watts S, Khallaayounde K, Bensefia R, Laamrani H, Gryseels B (1998) The study of human behavior and schistosomiasis transmission in an integrated area in Morocco. Soc Sci Med 6:755–765
World Health Organization (2018) Schistosomiasis. Fact sheet n. 115. http://www.who.int/mediacentre/factsheets/fs115/en/. Accessed 26 Mar 2018
World Health Organization (1990) Health education in the control of Schistosomiasis. WHO Library Cataloguing in Publication Data, Geneva
World Health Organization (2013) Regional office for Africa regional strategic plan for neglected tropical diseases in the African Region 2014–2020, Brazzaville
Wu J, Feng Z (2002) Mathematical models for schistosomiasis with delays and multiple definitive hosts. In: Mathematical approaches for emerging and reemerging infectious diseases: models, methods and theory (Minneapolis, MN, 1999), IMA volumes in mathematics and its applications, vol 126. Springer, New York, pp 215–229
Yang C, Wan X, Gao D, Wang J (2017) Impact of awareness programs on cholera dynamics: two modeling approaches. Bull Math Biol 79:2109–2131
Yingke L, Zhidong T, Ruan S, Mingtao L, Xiaomei F (2017) A mathematical model for the seasonal transmission of schistosomiasis in the lake and marshland regions of China. Math Biosci Eng 14:1279–1299
Yun S, Yuanguo Z (2016) The effect of time delays on transmission dynamics of schistosomiasis. Chaos Solitons Fract 91:360–371
Yan-Fang W, Ming-Tao L, Gui-Quan S (2016) Asymptotic analysis of schistosomiasis persistence in models with general functions. J Frankl Inst 353:4772–4784
Zeng H, Yang X, Meng S, Wang H, Tang X, Tang W, Zeng S, Jeschke S, Wang Y (2011) Awareness and knowledge of schistosomiasis infection and prevention in the “Three Gorges Dam” reservoir area: a cross-sectional study on local residents and health personnel. Acta Trop 120:238–244
Zhang X, Gao S, Cao H (2014) Threshold dynamics for a non-autonomous schistosomiasis model in a periodic environment. J Appl Math Comput 46:305–319
Zhang H, Harvim P, Georgescu P (2017) Preventing the spread of schistosomiasis in Ghana: possible outcomes of integrated optimal control strategies. J Biol Syst 25:625–655
Zhao X-Q (2003) Dynamical systems in population biology. Springer, New York
Zhao X-Q, Jing Z (1996) Global asymptotic behavior in some cooperative systems of functional-differential equations. Can Appl Math Q 4:421–444
Acknowledgements
The third author (BT) acknowledges the financial support of the University of Pretoria Senior Postdoctoral Program Grant (2018–2020). The authors are very grateful to the anonymous reviewers for the deep reports and the constructive suggestions which they made on their manuscript, that have immensely improved its quality.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Luz de Teresa.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendixes
Appendixes
1.1 Appendix A: Proof of Corollary 1
Proof
We are going to prove that \(\dfrac{\partial I^*_\mathrm{h}}{\partial \beta _\mathrm{M}} \le 0\). Recall the equilibrium equations:
In system (24), the fourth equation gives \(M^* = \dfrac{f(I^*_\mathrm{h})}{d_\mathrm{m}}\), from which the first and second equations yield:
and
Putting the two expressions above into the third equation of (24), yields another expression of \(v(I^*_\mathrm{h})\) in the form, one has:
Recalling from (1) that \(\beta _\mathrm{h}(I^*_\mathrm{h}) = \beta _0 - \beta _\mathrm{M} \widetilde{\beta _\mathrm{h}}(I^*_\mathrm{h})\), \(v(I^*_\mathrm{h})\) in (25) becomes:
Thus, the \(I^*_\mathrm{h}\)-component of the endemic equilibrium \(E^*\) is the solution of:
After differentiating both sides of (28) with respect to \(\beta _\mathrm{M}\) using the chain rule, follow by some rearrangements, one has:
where:
Have in mind that, from (26), one has:
Since f, g and \(\widetilde{\beta _\mathrm{h}}(I^*_\mathrm{h})\) are increasing, we have \( u'(I^*_\mathrm{h}), W(I^*_\mathrm{h}) \ge 0\), so that the right-hand side of (29) is negative and the expression into the big brackets on the left-hand side of (29) is positive. Hence, the proof is achieved.
1.2 Appendix B: A primer on graph theory and monotone dynamical systems
1.2.1 A primer on graph theory
Definition B1 (Berman and Plemmons 1979, page 29) For a \(n\times n\) matrix \( A= \left( a_{ij}\right) _{1\le i, j\le n}\), the directed graph G(A) consists of \(P_1,P_2, ... ,P_n\) vertices where an edge leads from \(P_i\), to \(P_j\) if and only if \(a_{ij}\ne 0\).
Definition B2 (Berman and Plemmons 1979, page 30) A directed graph G(A) is strongly connected if, for any ordered pair \((P_i,P_j), i\ne j\) of vertices of G(A), there exists a sequence of edges (a path) which leads from \(P_i\) to \(P_j\).
Theorem B1 (Berman and Plemmons 1979, Theorem (2.7), page 31) A matrix A is irreducible if and only if G(A) is strongly connected.
1.2.2 A primer on monotone dynamical systems
Consider an ordinary differential equation (ODE):
defined on a positively invariant set U. We denote by \(\Phi _t (x)\) the flow of (30). We assume, to avoid complications, that this is defined for any \(t\ge 0\) (this will be the case our system). We consider the standard partial order on \(\mathbb R^n\) and recall the following corresponding notations:
Definition B3 (Hirsch 1988; Iggidr et al. 2012) System (1) is called monotone if \(x \le y\) implies \(\Phi _t(x) \le \Phi _t(y)\).
Definition B4 (Hirsch 1985; Iggidr et al. 2012) System (2) is called strongly monotone if \( x < y \) implies \(\Phi _t(x) \ll \Phi _t(y)\) for any \(t > 0.\)
Theorem B4 ((Smith 1988; Sokolowa et al. 2015), Lemma 2.1) If f is \(C^1\), then System (1) is monotone if and only if the Jacobian of f is a Metzler matrix. Note that a Metzler matrix is a matrix whose off-diagonal terms are nonnegative.
Theorem B5 (Hirsch 1988; Iggidr et al. 2012) System (2) is strongly monotone if its Jacobian matrix is irreducible.
Definition B5 (Berman and Plemmons 1979, page 138) A matrix A has a regular splitting/decomposition if A has a representation \(A= M-N\), where \(M^{-1} \ge 0\), \(N \ge 0\).
Rights and permissions
About this article
Cite this article
Aziz-Alaoui, M.A., Lubuma, J.MS. & Tsanou, B. Prevalence-based modeling approach of schistosomiasis: global stability analysis and integrated control assessment. Comp. Appl. Math. 40, 24 (2021). https://doi.org/10.1007/s40314-021-01414-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40314-021-01414-9
Keywords
- Schistosomiasis
- Human behavior
- Prevalence-based modeling
- Integrated control
- Monotone system
- Global stability
- Numerical simulation