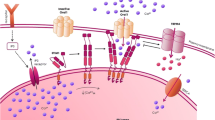
Abstract
Ion channels have major regulatory functions in living cells. Apart from their role in ion transport, they are responsible for cellular electrogenesis and excitability, and may also regulate tissue homeostasis. Although cancer is not officially classified as a channelopathy, it has been increasingly recognized that ion channel aberrations play an important role in virtually all cancer types. Ion channels can exert pro-tumorigenic activities due to genetic or epigenetic alterations, or as a response to molecular signals, such as growth factors, hormones, etc. Increasing evidence suggests that ion channels and pumps play a critical role in the regulation of prostate cancer cell proliferation, apoptosis evasion, migration, epithelial-to-mesenchymal transition, and angiogenesis. There is also evidence suggesting that ion channels might play a role in treatment failure in patients with prostate cancer. Hence, they represent promising targets for diagnosis, staging, and treatment, and their effects may be of particular significance for specific patient populations, including those undergoing anesthesia and surgery. In this article, the role of major types of ion channels involved in the development and progression of prostate cancer are reviewed. Identifying the underlying molecular mechanisms of the pro-tumorigenic effects of ion channels may potentially inform the development of novel therapeutic strategies to counter this malignancy.


Similar content being viewed by others
References
Jentsch TJ, Hübner CA, Fuhrmann JC. Ion channels: function unravelled by dysfunction. Nat Cell Biol. 2004;6:1039–47. https://doi.org/10.1038/ncb1104-1039.
Litan A, Langhans SA. Cancer as a channelopathy: ion channels and pumps in tumor development and progression. Front Cell Neurosci. 2015;9:86. https://doi.org/10.3389/fncel.2015.00086.
Lang F, Föller M, Lang K, Lang P, Ritter M, Vereninov A, Szabo I, Huber SM, Gulbins E. Cell volume regulatory ion channels in cell proliferation and cell death. Methods Enzymol. 2007;428:209–25. https://doi.org/10.1016/S0076-6879(07)28011-5.
Prevarskaya N, Skryma R, Shuba Y. Ion channels and the hallmarks of cancer. Trends Mol Med. 2010;16:107–21. https://doi.org/10.1016/j.molmed.2010.01.005.
Kischel P, Girault A, Rodat-Despoix L, Chamlali M, Radoslavova S, Abou Daya H, Lefebvre T, Foulon A, Rybarczyk P, Hague F, Dhennin-Duthille I, Gautier M, Ouadid-Ahidouch H. Ion channels: new actors playing in chemotherapeutic resistance. Cancers (Basel). 2019;11:376. https://doi.org/10.3390/cancers11030376.
Imbrici P, Liantonio A, Camerino GM, De Bellis M, Camerino C, Mele A, Giustino A, Pierno S, De Luca A, Tricarico D, Desaphy JF, Conte D. Therapeutic approaches to genetic ion channelopathies and perspectives in drug discovery. Front Pharmacol. 2016;7:121. https://doi.org/10.3389/fphar.2016.00121.
Lipscombe D, Lopez-Soto EJ. Epigenetic control of ion channel expression and cell-specific splicing in nociceptors: chronic pain mechanisms and potential therapeutic targets. Channels (Austin). 2021;15:156–64. https://doi.org/10.1080/19336950.2020.1860383.
Restrepo-Angulo I, Bañuelos C, Camacho J. Ion channel regulation by sex steroid hormones and vitamin D in cancer: a potential opportunity for cancer diagnosis and therapy. Front Pharmacol. 2020;11:152. https://doi.org/10.3389/fphar.2020.00152.
Fraser SP, Ozerlat-Gunduz I, Brackenbury WJ, Fitzgerald EM, Campbell TM, Coombes RC, Djamgoz MB. Regulation of voltage-gated sodium channel expression in cancer: hormones, growth factors and auto-regulation. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130105. https://doi.org/10.1098/rstb.2013.0105.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. https://doi.org/10.3322/caac.21654.
Siegel DA, O’Neil ME, Richards TB, Dowling NF, Weir HK. Prostate cancer incidence and survival, by stage and race/ethnicity—United States, 2001–2017. MMWR Morb Mortal Wkly Rep. 2020;69:1473–80. https://doi.org/10.15585/mmwr.mm6941a1.
Covas Moschovas M, Chew C, Bhat S, Sandri M, Rogers T, Dell’Oglio P, et al. Association between oncotype DX genomic prostate score and adverse tumor pathology after radical prostatectomy. Eur Urol Focus. 2022;8(2):418–24. https://doi.org/10.1016/j.euf.2021.03.015.
Wang Y, Yang Z. A Gleason score-related outcome model for human prostate cancer: a comprehensive study based on weighted gene co-expression network analysis. Cancer Cell Int. 2020;20:159. https://doi.org/10.1186/s12935-020-01230-x.
James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, Ritchie AWS, Amos CL, Gilson C, Jones RJ, Matheson D, Millman R, Attard G, Chowdhury S, Cross WR, Gillessen S, Parker CC, Russell JM, Berthold DR, Brawley C, Adab F, Aung S, Birtle AJ, Bowen J, Brock S, Chakraborti P, Ferguson C, Gale J, Gray E, Hingorani M, Hoskin PJ, Lester JF, Malik ZI, McKinna F, McPhail N, Money-Kyrle J, O’Sullivan J, Parikh O, Protheroe A, Robinson A, Srihari NN, Thomas C, Wagstaff J, Wylie J, Zarkar A, Parmar MKB, Sydes MR, STAMPEDE Investigators. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338–51. https://doi.org/10.1056/NEJMoa1702900.
Evans AJ. Treatment effects in prostate cancer. Mod Pathol. 2018;31:S110–21. https://doi.org/10.1038/modpathol.2017.158.
Prevarskaya N, Skryma R, Bidaux G, Flourakis M, Shuba Y. Ion channels in death and differentiation of prostate cancer cells. Cell Death Differ. 2007;14:1295–304. https://doi.org/10.1038/sj.cdd.4402162.
Yu H. Depolarization or hyperpolarization: emerging role of altered bioelectricity in breast cancer metastasis. EBioMedicine. 2022;76:103853. https://doi.org/10.1016/j.ebiom.2022.103853.
Fiorio Pla A, Munaron L. Functional properties of ion channels and transporters in tumour vascularization. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130103. https://doi.org/10.1098/rstb.2013.0103.
Wang Z. Roles of K+ channels in regulating tumour cell proliferation and apoptosis. Pflugers Arch. 2004;448:274–86. https://doi.org/10.1007/s00424-004-1258-5.
Kaczmarek LK, Aldrich RW, Chandy KG, Grissmer S, Wei AD, Wulff H. International union of basic and clinical pharmacology. C. Nomenclature and properties of calcium-activated and sodium-activated potassium channels. Pharmacol Rev. 2017;69:1–11. https://doi.org/10.1124/pr.116.012864.
Abdul M, Hoosein N. Expression and activity of potassium ion channels in human prostate cancer. Cancer Lett. 2002;186:99–105. https://doi.org/10.1016/s0304-3835(02)00348-8.
Laniado ME, Fraser SP, Djamgoz MB. Voltage-gated K(+) channel activity in human prostate cancer cell lines of markedly different metastatic potential: distinguishing characteristics of PC-3 and LNCaP cells. Prostate. 2001;46:262–74. https://doi.org/10.1002/1097-0045(20010301)46:4%3c262::aid-pros1032%3e3.0.co;2-f.
Bortner CD, Cidlowski JA. Ion channels and apoptosis in cancer. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130104. https://doi.org/10.1098/rstb.2013.0104.
Remillard CV, Yuan JX. Activation of K+ channels: an essential pathway in programmed cell death. Am J Physiol Lung Cell Mol Physiol. 2004;286:L49-67. https://doi.org/10.1152/ajplung.00041.2003.
Lallet-Daher H, Wiel C, Gitenay D, Navaratnam N, Augert A, Le Calvé B, Verbeke S, Carling D, Aubert S, Vindrieux D, Bernard D. Potassium channel KCNA1 modulates oncogene-induced senescence and transformation. Cancer Res. 2013;73:5253–65. https://doi.org/10.1158/0008-5472.CAN-12-3690.
Park HW, Song MS, Sim HJ, Ryu PD, Lee SY. The role of the voltage-gated potassium channel, Kv2.1 in prostate cancer cell migration. BMB Rep. 2021;54:130–5. https://doi.org/10.5483/BMBRep.2021.54.2.210.
Hu X, Wei L, Taylor TM, Wei J, Zhou X, Wang JA, Yu SP. Hypoxic preconditioning enhances bone marrow mesenchymal stem cell migration via Kv2.1 channel and FAK activation. Am J Physiol Cell Physiol. 2011;301:C362–72. https://doi.org/10.1152/ajpcell.00013.2010.
Abdul M, Hoosein N. Reduced Kv1.3 potassium channel expression in human prostate cancer. J Membr Biol. 2006;214:99–102. https://doi.org/10.1007/s00232-006-0065-7.
Teisseyre A, Palko-Labuz A, Sroda-Pomianek K, Michalak K. Voltage-gated potassium channel Kv1.3 as a target in therapy of cancer. Front Oncol. 2019;9:933. https://doi.org/10.3389/fonc.2019.00933.
Jiménez-Pérez L, Cidad P, Álvarez-Miguel I, Santos-Hipólito A, Torres-Merino R, Alonso E, de la Fuente MÁ, López-López JR, Pérez-García MT. Molecular determinants of Kv1.3 potassium channels-induced proliferation. J Biol Chem. 2016;291:3569–80. https://doi.org/10.1074/jbc.M115.678995.
Bloch M, Ousingsawat J, Simon R, Schraml P, Gasser TC, Mihatsch MJ, Kunzelmann K, Bubendorf L. KCNMA1 gene amplification promotes tumor cell proliferation in human prostate cancer. Oncogene. 2007;26:2525–34. https://doi.org/10.1038/sj.onc.1210036.
Gessner G, Schönherr K, Soom M, Hansel A, Asim M, Baniahmad A, Derst C, Hoshi T, Heinemann SH. BKCa channels activating at resting potential without calcium in LNCaP prostate cancer cells. J Membr Biol. 2005;208:229–40. https://doi.org/10.1007/s00232-005-0830-z.
Gackière F, Warnier M, Katsogiannou M, Derouiche S, Delcourt P, Dewailly E, Slomianny C, Humez S, Prevarskaya N, Roudbaraki M, Mariot P. Functional coupling between large-conductance potassium channels and Cav3.2 voltage-dependent calcium channels participates in prostate cancer cell growth. Biol Open. 2013;2:941–51. https://doi.org/10.1242/bio.20135215.
Bery F, Cancel M, Guéguinou M, Potier-Cartereau M, Vandier C, Chantôme A, Guibon R, Bruyère F, Fromont G, Mahéo K. Zeb1 and SK3 channel are up-regulated in castration-resistant prostate cancer and promote neuroendocrine differentiation. Cancers (Basel). 2021;13:2947. https://doi.org/10.3390/cancers13122947.
Lallet-Daher H, Roudbaraki M, Bavencoffe A, Mariot P, Gackière F, Bidaux G, Urbain R, Gosset P, Delcourt P, Fleurisse L, Slomianny C, Dewailly E, Mauroy B, Bonnal JL, Skryma R, Prevarskaya N. Intermediate-conductance Ca2+-activated K+ channels (IKCa1) regulate human prostate cancer cell proliferation through a close control of calcium entry. Oncogene. 2009;28:1792–806. https://doi.org/10.1038/onc.2009.25.
Ohya S, Kimura K, Niwa S, Ohno A, Kojima Y, Sasaki S, Kohri K, Imaizumi Y. Malignancy grade-dependent expression of K+-channel subtypes in human prostate cancer. J Pharmacol Sci. 2009;109:148–51. https://doi.org/10.1254/jphs.08208sc.
Innamaa A, Jackson L, Asher V, van Schalkwyk G, Warren A, Keightley A, Hay D, Bali A, Sowter H, Khan R. Expression and effects of modulation of the K2P potassium channels TREK-1 (KCNK2) and TREK-2 (KCNK10) in the normal human ovary and epithelial ovarian cancer. Clin Transl Oncol. 2013;15:910–8. https://doi.org/10.1007/s12094-013-1022-4.
Zhang GM, Wan FN, Qin XJ, Cao DL, Zhang HL, Zhu Y, Dai B, Shi GH, Ye DW. Prognostic significance of the TREK-1 K2P potassium channels in prostate cancer. Oncotarget. 2015;6:18460–8. https://doi.org/10.18632/oncotarget.3782.
Zúñiga R, Concha G, Cayo A, Cikutović-Molina R, Arevalo B, González W, Catalán MA, Zúñiga L. Withaferin A suppresses breast cancer cell proliferation by inhibition of the two-pore domain potassium (K2P9) channel TASK-3. Biomed Pharmacother. 2020;129:110383. https://doi.org/10.1016/j.biopha.2020.110383.
Pei L, Wiser O, Slavin A, Mu D, Powers S, Jan LY, Hoey T. Oncogenic potential of TASK3 (Kcnk9) depends on K+ channel function. Proc Natl Acad Sci USA. 2003;100:7803–7. https://doi.org/10.1073/pnas.1232448100.
Mao W, Zhang J, Körner H, Jiang Y, Ying S. The emerging role of voltage-gated sodium channels in tumor biology. Front Oncol. 2019;9:124. https://doi.org/10.3389/fonc.2019.00124.
Yu FH, Catterall WA. Overview of the voltage-gated sodium channel family. Genome Biol. 2003;4:207. https://doi.org/10.1186/gb-2003-4-3-207.
Meisler MH, O’Brien JE, Sharkey LM. Sodium channel gene family: epilepsy mutations, gene interactions and modifier effects. J Physiol. 2010;588:1841–8. https://doi.org/10.1113/jphysiol.2010.188482.
Namadurai S, Yereddi NR, Cusdin FS, Huang CL, Chirgadze DY, Jackson AP. A new look at sodium channel β subunits. Open Biol. 2015;5:140192. https://doi.org/10.1098/rsob.140192.
Cameron IL, Smith NK, Pool TB, Sparks RL. Intracellular concentration of sodium and other elements as related to mitogenesis and oncogenesis in vivo. Cancer Res. 1980;40:1493–500.
Carrithers MD, Chatterjee G, Carrithers LM, Offoha R, Iheagwara U, Rahner C, Graham M, Waxman SG. Regulation of podosome formation in macrophages by a splice variant of the sodium channel SCN8A. J Biol Chem. 2009;284:8114–26. https://doi.org/10.1074/jbc.M801892200.
Yang M, Kozminski DJ, Wold LA, Modak R, Calhoun JD, Isom LL, Brackenbury WJ. Therapeutic potential for phenytoin: targeting Na(v)1.5 sodium channels to reduce migration and invasion in metastatic breast cancer. Breast Cancer Res Treat. 2012;134:603–15. https://doi.org/10.1007/s10549-012-2102-9.
Yildirim S, Altun S, Gumushan H, Patel A, Djamgoz MBA. Voltage-gated sodium channel activity promotes prostate cancer metastasis in vivo. Cancer Lett. 2012;323:58–61. https://doi.org/10.1016/j.canlet.2012.03.036.
Rhana P, Trivelato Junior RR, Beirão PSL, Cruz JS, Rodrigues ALP. Is there a role for voltage-gated Na+ channels in the aggressiveness of breast cancer? Braz J Med Biol Res. 2017;50:e6011. https://doi.org/10.1590/1414-431X20176011.
Suy S, Hansen TP, Auto HD, Kallakury BV, Dailey V, Danner M, Macarthur L, Zhang Y, Miessau MJ, Collins SP, Brown ML. Expression of voltage-gated sodium channel Nav1.8 in human prostate cancer is associated with high histological grade. J Clin Exp Oncol. 2012. https://doi.org/10.4172/2324-9110.1000102. (Epub ahead of print).
Bennett ES, Smith BA, Harper JM. Voltage-gated Na+ channels confer invasive properties on human prostate cancer cells. Pflugers Arch. 2004;447:908–14. https://doi.org/10.1007/s00424-003-1205-x.
Brackenbury WJ, Djamgoz MB. Nerve growth factor enhances voltage-gated Na+ channel activity and Transwell migration in Mat-LyLu rat prostate cancer cell line. J Cell Physiol. 2007;210:602–8. https://doi.org/10.1002/jcp.20846.
Jansson KH, Lynch JE, Lepori-Bui N, Czymmek KJ, Duncan RL, Sikes RA. Overexpression of the VSSC-associated CAM, β-2, enhances LNCaP cell metastasis associated behavior. Prostate. 2012;72:1080–92. https://doi.org/10.1002/pros.21512.
Gagnon KB, Delpire E. Sodium transporters in human health and disease. Front Physiol. 2021;11:588664. https://doi.org/10.3389/fphys.2020.588664.
Persi E, Duran-Frigola M, Damaghi M, Roush WR, Aloy P, Cleveland JL, Gillies RJ, Ruppin E. Systems analysis of intracellular pH vulnerabilities for cancer therapy. Nat Commun. 2018;9:2997. https://doi.org/10.1038/s41467-018-05261-x.
Damaghi M, Wojtkowiak JW, Gillies RJ. pH sensing and regulation in cancer. Front Physiol. 2013;4:370. https://doi.org/10.3389/fphys.2013.00370.
Schwab A, Stock C. Ion channels and transporters in tumour cell migration and invasion. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130102. https://doi.org/10.1098/rstb.2013.0102.
Li Y, Zhou X, Sun SX. Hydrogen, bicarbonate, and their associated exchangers in cell volume regulation. Front Cell Dev Biol. 2021;9:683686. https://doi.org/10.3389/fcell.2021.683686.
Li X, Buckley B, Stoletov K, Jing Y, Ranson M, Lewis JD, Kelso M, Fliegel L. Roles of the Na+/H+ exchanger isoform 1 and urokinase in prostate cancer cell migration and invasion. Int J Mol Sci. 2021;22:13263. https://doi.org/10.3390/ijms222413263.
Steffan JJ, Snider JL, Skalli O, Welbourne T, Cardelli JA. Na+/H+ exchangers and RhoA regulate acidic extracellular pH-induced lysosome trafficking in prostate cancer cells. Traffic. 2009;10:737–53. https://doi.org/10.1111/j.1600-0854.2009.00904.x.
Hiraoka K, Miyazaki H, Niisato N, Iwasaki Y, Kawauchi A, Miki T, Marunaka Y. Chloride ion modulates cell proliferation of human androgen-independent prostatic cancer cell. Cell Physiol Biochem. 2010;25:379–88. https://doi.org/10.1159/000303042.
Li JM, Lee S, Zafar R, Shin E, Choi I. Sodium bicarbonate transporter NBCe1 regulates proliferation and viability of human prostate cancer cells LNCaP and PC3. Oncol Rep. 2021;46:129. https://doi.org/10.3892/or.2021.8080.
Mignen O, Constantin B, Potier-Cartereau M, Penna A, Gautier M, Guéguinou M, Renaudineau Y, Shoji KF, Félix R, Bayet E, Buscaglia P, Debant M, Chantôme A, Vandier C. Constitutive calcium entry and cancer: updated views and insights. Eur Biophys J. 2017;46:395–413. https://doi.org/10.1007/s00249-017-1216-8.
Tajada S, Villalobos C. Calcium permeable channels in cancer hallmarks. Front Pharmacol. 2020;11:968. https://doi.org/10.3389/fphar.2020.00968.
Patergnani S, Danese A, Bouhamida E, Aguiari G, Previati M, Pinton P, Giorgi C. Various aspects of calcium signaling in the regulation of apoptosis, autophagy, cell proliferation, and cancer. Int J Mol Sci. 2020;21:8323. https://doi.org/10.3390/ijms21218323.
Qi H, Li X, Jin Z, Simmen T, Shuai J. The oscillation amplitude, not the frequency of cytosolic calcium, regulates apoptosis induction. iScience. 2020;23:101671. https://doi.org/10.1016/j.isci.2020.101671.
Ardura JA, Álvarez-Carrión L, Gutiérrez-Rojas I, Alonso V. Role of calcium signaling in prostate cancer progression: effects on cancer hallmarks and bone metastatic mechanisms. Cancers (Basel). 2020;12:1071. https://doi.org/10.3390/cancers12051071.
Sanchez-Collado J, Jardin I, López JJ, Ronco V, Salido GM, Dubois C, Prevarskaya N, Rosado JA. Role of Orai3 in the pathophysiology of cancer. Int J Mol Sci. 2021;22:11426. https://doi.org/10.3390/ijms222111426.
Dubois C, Vanden Abeele F, Lehen’kyi V, Gkika D, Guarmit B, Lepage G, Slomianny C, Borowiec AS, Bidaux G, Benahmed M, Shuba Y, Prevarskaya N. Remodeling of channel-forming ORAI proteins determines an oncogenic switch in prostate cancer. Cancer Cell. 2014;26:19–32. https://doi.org/10.1016/j.ccr.2014.04.025.
Raphaël M, Lehen’kyi V, Vandenberghe M, Beck B, Khalimonchyk S, Vanden Abeele F, Farsetti L, Germain E, Bokhobza A, Mihalache A, Gosset P, Romanin C, Clézardin P, Skryma R, Prevarskaya N. TRPV6 calcium channel translocates to the plasma membrane via Orai1-mediated mechanism and controls cancer cell survival. Proc Natl Acad Sci USA. 2014;111:E3870–9. https://doi.org/10.1073/pnas.1413409111.
Lehen’kyi V, Flourakis M, Skryma R, Prevarskaya N. TRPV6 channel controls prostate cancer cell proliferation via Ca(2+)/NFAT-dependent pathways. Oncogene. 2007;26:7380–5. https://doi.org/10.1038/sj.onc.1210545.
Zhang L, Barritt GJ. Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells. Cancer Res. 2004;64:8365–73. https://doi.org/10.1158/0008-5472.CAN-04-2146.
Genovesi S, Moro R, Vignoli B, De Felice D, Canossa M, Montironi R, Carbone FG, Barbareschi M, Lunardi A, Alaimo A. Trpm8 expression in human and mouse castration resistant prostate adenocarcinoma paves the way for the preclinical development of TRPM8-based targeted therapies. Biomolecules. 2022;12:193. https://doi.org/10.3390/biom12020193.
Yang ZH, Wang XH, Wang HP, Hu LQ. Effects of TRPM8 on the proliferation and motility of prostate cancer PC-3 cells. Asian J Androl. 2009;11:157–65. https://doi.org/10.1038/aja.2009.1.
Alaimo A, Lorenzoni M, Ambrosino P, Bertossi A, Bisio A, Macchia A, Zoni E, Genovesi S, Cambuli F, Foletto V, De Felice D, Soldovieri MV, Mosca I, Gandolfi F, Brunelli M, Petris G, Cereseto A, Villarroel A, Thalmann G, Carbone FG, Kruithof-de Julio M, Barbareschi M, Romanel A, Taglialatela M, Lunardi A. Calcium cytotoxicity sensitizes prostate cancer cells to standard-of-care treatments for locally advanced tumors. Cell Death Dis. 2020;11:1039. https://doi.org/10.1038/s41419-020-03256-5.
Di Donato M, Ostacolo C, Giovannelli P, Di Sarno V, Monterrey IMG, Campiglia P, et al. Therapeutic potential of TRPM8 antagonists in prostate cancer. Sci Rep. 2021;11:23232. https://doi.org/10.1038/s41598-021-02675-4.
Di Sarno V, Giovannelli P, Medina-Peris A, Ciaglia T, Di Donato M, Musella S, et al. New TRPM8 blockers exert anticancer activity over castration-resistant prostate cancer models. Eur J Med Chem. 2022;238:114435. https://doi.org/10.1016/j.ejmech.2022.114435.
Gkika D, Lemonnier L, Shapovalov G, Gordienko D, Poux C, Bernardini M, Bokhobza A, Bidaux G, Degerny C, Verreman K, Guarmit B, Benahmed M, de Launoit Y, Bindels RJ, Fiorio Pla A, Prevarskaya N. TRP channel-associated factors are a novel protein family that regulates TRPM8 trafficking and activity. J Cell Biol. 2015;208:89–107. https://doi.org/10.1083/jcb.201402076.
Kuchay S, Giorgi C, Simoneschi D, Pagan J, Missiroli S, Saraf A, Florens L, Washburn MP, Collazo-Lorduy A, Castillo-Martin M, Cordon-Cardo C, Sebti SM, Pinton P, Pagano M. PTEN counteracts FBXL2 to promote IP3R3- and Ca2+-mediated apoptosis limiting tumour growth. Nature. 2017;546:554–8. https://doi.org/10.1038/nature22965.
Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–65. https://doi.org/10.1038/nrm1150.
Hall M, Todd B, Allen ED Jr, Nguyen N, Kwon YJ, Nguyen V, Hearne JL, Martin-Caraballo M. Androgen receptor signaling regulates T-type Ca2+ channel expression and neuroendocrine differentiation in prostate cancer cells. Am J Cancer Res. 2018;8:732–47.
Silvestri R, Pucci P, Venalainen E, Matheou C, Mather R, Chandler S, Aceto R, Rigas SH, Wang Y, Rietdorf K, Bootman MD, Crea F. T-type calcium channels drive the proliferation of androgen-receptor negative prostate cancer cells. Prostate. 2019;79:1580–6. https://doi.org/10.1002/pros.23879.
O’Reilly D, Downing T, Kouba S, Potier-Cartereau M, McKenna DJ, Vandier C, Buchanan PJ. CaV1.3 enhanced store operated calcium promotes resistance to androgen deprivation in prostate cancer. Cell Calcium. 2022;103:102554. https://doi.org/10.1016/j.ceca.2022.102554.
Chen R, Zeng X, Zhang R, Huang J, Kuang X, Yang J, Liu J, Tawfik O, Thrasher JB, Li B. Cav1.3 channel α1D protein is overexpressed and modulates androgen receptor transactivation in prostate cancers. Urol Oncol. 2014;32:524–36. https://doi.org/10.1016/j.urolonc.2013.05.011.
Warnier M, Roudbaraki M, Derouiche S, Delcourt P, Bokhobza A, Prevarskaya N, Mariot P. CACNA2D2 promotes tumorigenesis by stimulating cell proliferation and angiogenesis. Oncogene. 2015;34:5383–94. https://doi.org/10.1038/onc.2014.467.
Wang Y, Yue D, Li K, Liu YL, Ren CS, Wang P. The role of TRPC6 in HGF-induced cell proliferation of human prostate cancer DU145 and PC3 cells. Asian J Androl. 2010;12:841–52. https://doi.org/10.1038/aja.2010.85.
Sagredo AI, Sagredo EA, Cappelli C, Báez P, Andaur RE, Blanco C, Tapia JC, Echeverría C, Cerda O, Stutzin A, Simon F, Marcelain K, Armisén R. TRPM4 regulates Akt/GSK3-β activity and enhances β-catenin signaling and cell proliferation in prostate cancer cells. Mol Oncol. 2018;12:151–65. https://doi.org/10.1002/1878-0261.12100.
Berg KD, Soldini D, Jung M, Dietrich D, Stephan C, Jung K, Dietel M, Vainer B, Kristiansen G. TRPM4 protein expression in prostate cancer: a novel tissue biomarker associated with risk of biochemical recurrence following radical prostatectomy. Virchows Arch. 2016;468:345–55. https://doi.org/10.1007/s00428-015-1880-y.
Stokłosa P, Kappel S, Peinelt C. A novel role of the TRPM4 ion channel in exocytosis. Cells. 2022;11:1793. https://doi.org/10.3390/cells11111793.
Zeng X, Sikka SC, Huang L, Sun C, Xu C, Jia D, Abdel-Mageed AB, Pottle JE, Taylor JT, Li M. Novel role for the transient receptor potential channel TRPM2 in prostate cancer cell proliferation. Prostate Cancer Prostatic Dis. 2010;13:195–201. https://doi.org/10.1038/pcan.2009.55.
Breuksch I, Weinert M, Brenner W. The role of extracellular calcium in bone metastasis. J Bone Oncol. 2016;5:143–5. https://doi.org/10.1016/j.jbo.2016.06.004.
Sun Y, Selvaraj S, Varma A, Derry S, Sahmoun AE, Singh BB. Increase in serum Ca2+/Mg2+ ratio promotes proliferation of prostate cancer cells by activating TRPM7 channels. J Biol Chem. 2013;288:255–63. https://doi.org/10.1074/jbc.M112.393918.
Bernardini M, Brossa A, Chinigo G, Grolez GP, Trimaglio G, Allart L, Hulot A, Marot G, Genova T, Joshi A, Mattot V, Fromont G, Munaron L, Bussolati B, Prevarskaya N, Fiorio Pla A, Gkika D. Transient receptor potential channel expression signatures in tumor-derived endothelial cells: functional roles in prostate cancer angiogenesis. Cancers (Basel). 2019;11:956. https://doi.org/10.3390/cancers11070956.
Sun Y, Schaar A, Sukumaran P, Dhasarathy A, Singh BB. TGFβ-induced epithelial-to-mesenchymal transition in prostate cancer cells is mediated via TRPM7 expression. Mol Carcinog. 2018;57:752–61. https://doi.org/10.1002/mc.22797.
Yang F, Cai J, Zhan H, Situ J, Li W, Mao Y, Luo Y. Suppression of TRPM7 inhibited hypoxia-induced migration and invasion of androgen-independent prostate cancer cells by enhancing RACK1-mediated degradation of HIF-1α. Oxid Med Cell Longev. 2020;2020:6724810. https://doi.org/10.1155/2020/6724810.
Monet M, Lehen’kyi V, Gackiere F, Firlej V, Vandenberghe M, Roudbaraki M, Gkika D, Pourtier A, Bidaux G, Slomianny C, Delcourt P, Rassendren F, Bergerat JP, Ceraline J, Cabon F, Humez S, Prevarskaya N. Role of cationic channel TRPV2 in promoting prostate cancer migration and progression to androgen resistance. Cancer Res. 2010;70:1225–35. https://doi.org/10.1158/0008-5472.CAN-09-2205.
Wang D, Li X, Liu J, Li J, Li LJ, Qiu MX. Effects of TRPC6 on invasibility of low-differentiated prostate cancer cells. Asian Pac J Trop Med. 2014;7:44–7. https://doi.org/10.1016/S1995-7645(13)60190-X.
Chantôme A, Potier-Cartereau M, Clarysse L, Fromont G, Marionneau-Lambot S, Guéguinou M, Pagès JC, Collin C, Oullier T, Girault A, Arbion F, Haelters JP, Jaffrès PA, Pinault M, Besson P, Joulin V, Bougnoux P, Vandier C. Pivotal role of the lipid Raft SK3-Orai1 complex in human cancer cell migration and bone metastases. Cancer Res. 2013;73:4852–61. https://doi.org/10.1158/0008-5472.CAN-12-4572.
Ringstad N, Abe N, Horvitz HR. Ligand-gated chloride channels are receptors for biogenic amines in C. elegans. Science. 2009;325:96–100. https://doi.org/10.1126/science.1169243.
Poroca DR, Pelis RM, Chappe VM. ClC channels and transporters: structure, physiological functions, and implications in human chloride channelopathies. Front Pharmacol. 2017;8:151. https://doi.org/10.3389/fphar.2017.00151.
Peretti M, Angelini M, Savalli N, Florio T, Yuspa SH, Mazzanti M. Chloride channels in cancer: focus on chloride intracellular channel 1 and 4 (CLIC1 AND CLIC4) proteins in tumor development and as novel therapeutic targets. Biochim Biophys Acta. 2015;1848:2523–31. https://doi.org/10.1016/j.bbamem.2014.12.012.
Voss FK, Ullrich F, Münch J, Lazarow K, Lutter D, Mah N, Andrade-Navarro MA, von Kries JP, Stauber T, Jentsch TJ. Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science. 2014;344:634–8. https://doi.org/10.1126/science.1252826.
Stakaitytė G, Nwogu N, Lippiat JD, Blair GE, Poterlowicz K, Boyne JR, Macdonald A, Mankouri J, Whitehouse A. The cellular chloride channels CLIC1 and CLIC4 contribute to virus-mediated cell motility. J Biol Chem. 2018;293:4582–90. https://doi.org/10.1074/jbc.RA117.001343.
Okada Y, Maeno E, Shimizu T, Dezaki K, Wang J, Morishima S. Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD). J Physiol. 2001;532:3–16. https://doi.org/10.1111/j.1469-7793.2001.0003g.x.
Vanoverberghe K, Vanden Abeele F, Mariot P, Lepage G, Roudbaraki M, Bonnal JL, Mauroy B, Shuba Y, Skryma R, Prevarskaya N. Ca2+ homeostasis and apoptotic resistance of neuroendocrine-differentiated prostate cancer cells. Cell Death Differ. 2004;11:321–30. https://doi.org/10.1038/sj.cdd.4401375.
Lemonnier L, Lazarenko R, Shuba Y, Thebault S, Roudbaraki M, Lepage G, Prevarskaya N, Skryma R. Alterations in the regulatory volume decrease (RVD) and swelling-activated Cl− current associated with neuroendocrine differentiation of prostate cancer epithelial cells. Endocr Relat Cancer. 2005;12:335–49. https://doi.org/10.1677/erc.1.00898.
Lemonnier L, Prevarskaya N, Shuba Y, Vanden Abeele F, Nilius B, Mazurier J, Skryma R. Ca2+ modulation of volume-regulated anion channels: evidence for colocalization with store-operated channels. FASEB J. 2002;16:222–4. https://doi.org/10.1096/fj.01-0383fje.
Seo Y, Ryu K, Park J, Jeon DK, Jo S, Lee HK, Namkung W. Inhibition of ANO1 by luteolin and its cytotoxicity in human prostate cancer PC-3 cells. PLoS One. 2017;12:e0174935. https://doi.org/10.1371/journal.pone.0174935.
Tian Y, Guan Y, Jia Y, Meng Q, Yang J. Chloride intracellular channel 1 regulates prostate cancer cell proliferation and migration through the MAPK/ERK pathway. Cancer Biother Radiopharm. 2014;29:339–44. https://doi.org/10.1089/cbr.2014.1666.
Themistocleous SC, Yiallouris A, Tsioutis C, Zaravinos A, Johnson EO, Patrikios I. Clinical significance of P-class pumps in cancer. Oncol Lett. 2021;22:658. https://doi.org/10.3892/ol.2021.12919.
Banerjee M, Cui X, Li Z, Yu H, Cai L, Jia X, He D, Wang C, Gao T, Xie Z. Na/K-ATPase Y260 phosphorylation-mediated Src regulation in control of aerobic glycolysis and tumor growth. Sci Rep. 2018;8:12322. https://doi.org/10.1038/s41598-018-29995-2.
Banerjee M, Li Z, Gao Y, Lai F, Huang M, Zhang Z, Cai L, Sanabria J, Gao T, Xie Z, Pierre SV. Inverse agonism at the Na/K-ATPase receptor reverses EMT in prostate cancer cells. Prostate. 2021;81:667–82. https://doi.org/10.1002/pros.24144.
Grzmil M, Voigt S, Thelen P, Hemmerlein B, Helmke K, Burfeind P. Up-regulated expression of the MAT-8 gene in prostate cancer and its siRNA-mediated inhibition of expression induces a decrease in proliferation of human prostate carcinoma cells. Int J Oncol. 2004;24:97–105.
Streif D, Iglseder E, Hauser-Kronberger C, Fink KG, Jakab M, Ritter M. Expression of the non-gastric H+/K+ ATPase ATP12A in normal and pathological human prostate tissue. Cell Physiol Biochem. 2011;28:1287–94. https://doi.org/10.1159/000335860.
Whitton B, Okamoto H, Rose-Zerilli M, Packham G, Crabb SJ. V-ATPase inhibition decreases mutant androgen receptor activity in castrate-resistant prostate cancer. Mol Cancer Ther. 2021;20:739–48. https://doi.org/10.1158/1535-7163.MCT-20-0662.
Licon-Munoz Y, Michel V, Fordyce CA, Parra KJ. F-actin reorganization by V-ATPase inhibition in prostate cancer. Biol Open. 2017;6:1734–44. https://doi.org/10.1242/bio.028837.
Denmeade SR, Jakobsen CM, Janssen S, Khan SR, Garrett ES, Lilja H, Christensen SB, Isaacs JT. Prostate-specific antigen-activated thapsigargin prodrug as targeted therapy for prostate cancer. J Natl Cancer Inst. 2003;95:990–1000. https://doi.org/10.1093/jnci/95.13.990.
Brogi E, Forfori F. Anesthesia and cancer recurrence: an overview. J Anesth Analg Crit Care. 2022;2:33. https://doi.org/10.1186/s44158-022-00060-9.
Lee BM, Singh Ghotra V, Karam JA, Hernandez M, Pratt G, Cata JP. Regional anesthesia/analgesia and the risk of cancer recurrence and mortality after prostatectomy: a meta-analysis. Pain Manag. 2015;5:387–95. https://doi.org/10.2217/pmt.15.30.
Chalkias A, Spyropoulos V, Georgiou G, Laou E, Koutsovasilis A, Pantazopoulos I, Kolonia K, Vrakas S, Papalois A, Demeridou S, Gourgoulianis K, Dontas I, Kaparos G, Baka S, Xanthos T. Baseline values and kinetics of IL-6, procalcitonin, and TNF-α in landrace-large white swine anesthetized with propofol-based total intravenous anesthesia. Biomed Res Int. 2021;2021:6672573. https://doi.org/10.1155/2021/6672573.
Chalkias A, Barreto EF, Laou E, Kolonia K, Scheetz MH, Gourgoulianis K, Pantazopoulos I, Xanthos T. A critical appraisal of the effects of anesthetics on immune-system modulation in critically ill patients with COVID-19. Clin Ther. 2021;43:e57–70. https://doi.org/10.1016/j.clinthera.2021.01.004.
Laou E, Papagiannakis N, Tsiaka A, Tsapournioti S, Chatzikallinikidis K, Mantzaflaras G, Karadontas I, Eugen-Olsen J, Chalkias A. Soluble urokinase receptor levels are not affected by the systemic inflammatory response to anesthesia and operative trauma. Eur Surg Res. 2022. https://doi.org/10.1159/000524433. (Epub ahead of print).
Zhang Y, Jing Y, Pan R, Ding K, Chen R, Meng Q. Mechanisms of cancer inhibition by local anesthetics. Front Pharmacol. 2021;12:770694. https://doi.org/10.3389/fphar.2021.770694.
Lirk P, Berger R, Hollmann MW, Fiegl H. Lidocaine time- and dose-dependently demethylates deoxyribonucleic acid in breast cancer cell lines in vitro. Br J Anaesth. 2012;109:200–7. https://doi.org/10.1093/bja/aes128.
Baptista-Hon DT, Robertson FM, Robertson GB, Owen SJ, Rogers GW, Lydon EL, Lee NH, Hales TG. Potent inhibition by ropivacaine of metastatic colon cancer SW620 cell invasion and NaV1.5 channel function. Br J Anaesth. 2014;113(Suppl 1):i39–48. https://doi.org/10.1093/bja/aeu104.
Grandhi RK, Perona B. Mechanisms of action by which local anesthetics reduce cancer recurrence: a systematic review. Pain Med. 2020;21:401–14. https://doi.org/10.1093/pm/pnz139.
Mycielska ME, Fraser SP, Szatkowski M, Djamgoz MB. Contribution of functional voltage-gated Na+ channel expression to cell behaviors involved in the metastatic cascade in rat prostate cancer: II. Secretory membrane activity. J Cell Physiol. 2003;195:461–9. https://doi.org/10.1002/jcp.10265.
Gould HJ 3rd, Norleans J, Ward TD, Reid C, Paul D. Selective lysis of breast carcinomas by simultaneous stimulation of sodium channels and blockade of sodium pumps. Oncotarget. 2018;9:15606–15. https://doi.org/10.18632/oncotarget.24581.
Xuan W, Zhao H, Hankin J, Chen L, Yao S, Ma D. Local anesthetic bupivacaine induced ovarian and prostate cancer apoptotic cell death and underlying mechanisms in vitro. Sci Rep. 2016;6:26277. https://doi.org/10.1038/srep26277.
Liu H, Dilger JP, Lin J. The role of transient receptor potential melastatin 7 (TRPM7) in cell viability: a potential target to suppress breast cancer cell cycle. Cancers (Basel). 2020;12:131. https://doi.org/10.3390/cancers12010131.
Jiang Y, Gou H, Zhu J, Tian S, Yu L. Lidocaine inhibits the invasion and migration of TRPV6-expressing cancer cells by TRPV6 downregulation. Oncol Lett. 2016;12:1164–70. https://doi.org/10.3892/ol.2016.4709.
Batra S, Alenfall J. Characterization of peripheral benzodiazepine receptors in rat prostatic adenocarcinoma. Prostate. 1994;24:269–78. https://doi.org/10.1002/pros.2990240509.
Katz Y, Amiri Z, Weizman A, Gavish M. Identification and distribution of peripheral benzodiazepine binding sites in male rat genital tract. Biochem Pharmacol. 1990;40:817–20. https://doi.org/10.1016/0006-2952(90)90321-b.
Camins A, Sureda FX, Camarasa J, Escubedo E. Specific binding sites for [3H]Ro 5–4864 in rat prostate and seminal vesicle. Gen Pharmacol. 1992;23:381–4. https://doi.org/10.1016/0306-3623(92)90098-5.
Batra S, Alenfall J. Orchiectomy upregulates rabbit prostate peripheral benzodiazepine receptors. Life Sci. 1992;51:1211–5. https://doi.org/10.1016/0024-3205(92)90358-v.
Celsi F, Pizzo P, Brini M, Leo S, Fotino C, Pinton P, Rizzuto R. Mitochondria, calcium and cell death: a deadly triad in neurodegeneration. Biochim Biophys Acta. 2009;1787:335–44. https://doi.org/10.1016/j.bbabio.2009.02.021.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this work.
Conflict of interest
Minas Sakellakis and Athanasios Chalkias report no financial or non-financial competing interests that are directly or indirectly related to this manuscript.
Ethics approval
Not applicable.
Consent (participate and publication)
Not applicable.
Author contributions
MS conceptualized the review, reviewed the literature, and drafted and critically reviewed the manuscript. AC reviewed the literature, and drafted and critically reviewed the manuscript. Both authors approved the final version of the manuscript.
Data availability statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Code availability
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sakellakis, M., Chalkias, A. The Role οf Ion Channels in the Development and Progression of Prostate Cancer. Mol Diagn Ther 27, 227–242 (2023). https://doi.org/10.1007/s40291-022-00636-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-022-00636-9