Abstract
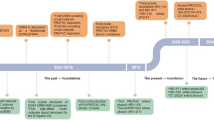
Proteolysis-targeting chimeras (PROTACs) are heterobifunctional chemicals that degrade proteins at the post-translational level, which represent an emerging therapeutic modality to fight cancer and other diseases. Although several PROTACs have now entered clinical trials, potential off-tissue side effects have resulted from nonspecific accumulation at non-cancerous sites after systemic administration, and this remains a major challenge. To this end, in the past 3 years, activatable PROTACs whose activity can only be launched on demand have gained tremendous momentum. In this review, we provide an overview of these new smart activatable PROTACs, which exert protein degradation action only in response to internal or external stimuli. We categorize these activatable PROTACs according to their activation mechanism contributed by different stimuli, including reduction-activatable, hypoxia-activatable, and enzyme-activatable PROTACs and photo-caged or photo-switchable PROTACs. The use of stimuli-responsive chemical blocks in these activatable PROTACs allows local activation of the antitumor effects while reducing the incidence of off-site side effects for precision cancer therapy. The design principle and category of smart PROTACs are introduced along with an overview of their therapeutic prospects and challenges.






Similar content being viewed by others
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33.
Wyld L, Audisio RA, Poston GJ. The evolution of cancer surgery and future perspectives. Nat Rev Clin Oncol. 2015;12(2):115–24.
Delaney G, Jacob S, Featherstone C, Barton M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer. 2005;104(6):1129–37.
Chabner BA, Roberts TG Jr. Timeline: chemotherapy and the war on cancer. Nat Rev Cancer. 2005;5(1):65–72.
Huang M, Shen A, Ding J, Geng M. Molecularly targeted cancer therapy: some lessons from the past decade. Trends Pharmacol Sci. 2014;35(1):41–50.
Xin YuJ, Hodge JP, Oliva C, Neftelinov ST, Hubbard-Lucey VM, Tang J. Trends in clinical development for PD-1/PD-L1 inhibitors. Nat Rev Drug Discov. 2020;19(3):163–4.
Sankar K, Gadgeel SM, Qin A. Molecular therapeutic targets in non-small cell lung cancer. Expert Rev Anticancer Ther. 2020;20(8):647–61.
Dale B, Cheng M, Park KS, Kaniskan HU, Xiong Y, Jin J. Advancing targeted protein degradation for cancer therapy. Nat Rev Cancer. 2021;21(10):638–54.
Pettersson M, Crews CM. PROteolysis TArgeting Chimeras (PROTACs): past, present and future. Drug Discov Today Technol. 2019;31:15–27.
Paiva SL, Crews CM. Targeted protein degradation: elements of PROTAC design. Curr Opin Chem Biol. 2019;50:111–9.
Lai AC, Crews CM. Induced protein degradation: an emerging drug discovery paradigm. Nat Rev Drug Discov. 2017;16(2):101–14.
Raina K, Crews CM. Targeted protein knockdown using small molecule degraders. Curr Opin Chem Biol. 2017;39:46–53.
Salami J, Crews CM. Waste disposal: an attractive strategy for cancer therapy. Science. 2017;355(6330):1163–7.
Shimokawa K, Shibata N, Sameshima T, Miyamoto N, Ujikawa O, Nara H, et al. Targeting the allosteric site of oncoprotein BCR-ABL as an alternative strategy for effective target protein degradation. ACS Med Chem Lett. 2017;8(10):1042–7.
Bond MJ, Chu L, Nalawansha DA, Li K, Crews CM. Targeted degradation of oncogenic KRAS(G12C) by VHL-recruiting PROTACs. ACS Cent Sci. 2020;6(8):1367–75.
Zhou H, Bai L, Xu R, Zhao Y, Chen J, McEachern D, et al. Structure-based discovery of SD-36 as a potent, selective, and efficacious PROTAC degrader of STAT3 protein. J Med Chem. 2019;62(24):11280–300.
Békés M, Langley DR, Crews CM. PROTAC targeted protein degraders: the past is prologue. Nat Rev Drug Discov. 2022;21(3):181–200.
Moreau K, Coen M, Zhang AX, Pachl F, Castaldi MP, Dahl G, et al. Proteolysis-targeting chimeras in drug development: a safety perspective. Br J Pharmacol. 2020;177(8):1709–18.
Raina K, Lu J, Qian Y, Altieri M, Gordon D, Rossi A, et al. PROTAC-induced BET protein degradation as a therapy for castration-resistant prostate cancer. Proc Natl Acad Sci USA. 2016;113(26):7124–9.
Jan M, Sperling AS, Ebert BL. Cancer therapies based on targeted protein degradation: lessons learned with lenalidomide. Nat Rev Clin Oncol. 2021;18(7):401–17.
Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501(7467):346–54.
Bansal A, Simon MC. Glutathione metabolism in cancer progression and treatment resistance. J Cell Biol. 2018;217(7):2291–8.
Holmström KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014;15(6):411–21.
Chen H, Liu J, Kaniskan H, Wei W, Jin J. Folate-guided protein degradation by immunomodulatory imide drug-based molecular glues and proteolysis targeting chimeras. J Med Chem. 2021;64(16):12273–85.
Zhang C, Han XR, Yang X, Jiang B, Liu J, Xiong Y, et al. Proteolysis targeting chimeras (PROTACs) of anaplastic lymphoma kinase (ALK). Eur J Med Chem. 2018;151:304–14.
Fischer ES, Bohm K, Lydeard JR, Yang H, Stadler MB, Cavadini S, et al. Structure of the DDB1-CRBN E3 ubiquitin ligase in complex with thalidomide. Nature. 2014;512(7512):49–53.
Gadd MS, Testa A, Lucas X, Chan KH, Chen W, Lamont DJ, et al. Structural basis of PROTAC cooperative recognition for selective protein degradation. Nat Chem Biol. 2017;13(5):514–21.
He S, Gao F, Ma J, Ma H, Dong G, Sheng C. Aptamer-PROTAC conjugates (APCs) for tumor-specific targeting in breast cancer. Angew Chem Int Ed. 2021;60(43):23299–305.
Dragovich PS, Pillow TH, Blake RA, Sadowsky JD, Adaligil E, Adhikari P, et al. Antibody-mediated delivery of chimeric BRD4 degraders. Part 2: improvement of in vitro antiproliferation activity and in vivo antitumor efficacy. J Med Chem. 2021;64(5):2576–607.
Dragovich PS, Pillow TH, Blake RA, Sadowsky JD, Adaligil E, Adhikari P, et al. Antibody-mediated delivery of chimeric BRD4 degraders. Part 1: exploration of antibody linker, payload loading, and payload molecular properties. J Med Chem. 2021;64(5):2534–75.
Pillow TH, Adhikari P, Blake RA, Chen J, Del Rosario G, Deshmukh G, et al. Antibody conjugation of a chimeric BET degrader enables in vivo activity. ChemMedChem. 2020;15(1):17–25.
Dragovich PS, Adhikari P, Blake RA, Blaquiere N, Chen J, Cheng YX. Antibody-mediated delivery of chimeric protein degraders which target estrogen receptor alpha (ERalpha). Bioorg Med Chem Lett. 2020;30(4): 126907.
Drago JZ, Modi S, Chandarlapaty S. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat Rev Clin Oncol. 2021;18(6):327–44.
Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11(6):393–410.
Cheng W, Li S, Wen X, Han S, Wang S, Wei H, et al. Development of hypoxia-activated PROTAC exerting a more potent effect in tumor hypoxia than in normoxia. Chem Commun. 2021;57(95):12852–5.
Liang SL, Chan DW. Enzymes and related proteins as cancer biomarkers: a proteomic approach. Clin Chim Acta. 2007;381(1):93–7.
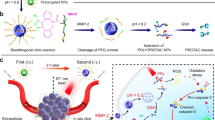
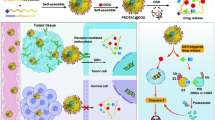
Zhang C, Zeng Z, Cui D, He S, Jiang Y, Li J, et al. Semiconducting polymer nano-PROTACs for activatable photo-immunometabolic cancer therapy. Nat Commun. 2021;12(1):2934.
Zhang C, He S, Zeng Z, Cheng P, Pu K. Smart nano-PROTACs reprogram tumor microenvironment for activatable photo-metabolic cancer immunotherapy. Angew Chem Int Ed. 2021;2021:e202114957.
Liu J, Chen H, Liu Y, Shen Y, Meng F, Kaniskan H, et al. Cancer selective target degradation by folate-caged PROTACs. J Am Chem Soc. 2021;143(19):7380–7.
Maneiro MA, Forte N, Shchepinova MM, Kounde CS, Chudasama V, Baker JR, et al. Antibody-PROTAC conjugates enable HER2-dependent targeted protein degradation of BRD4. ACS Chem Biol. 2020;15(6):1306–12.
Liang C, Zheng Q, Luo T, Cai W, Mao L, Wang M. Enzyme-catalyzed activation of pro-PROTAC for cell-selective protein degradation. CCS Chem. 2022. https://doi.org/10.31635/ccschem.022.202101529.
Li J, Pu K. Development of organic semiconducting materials for deep-tissue optical imaging, phototherapy and photoactivation. Chem Soc Rev. 2019;48(1):38–71.
Zeng S, Zhang H, Shen Z, Huang W. Photopharmacology of proteolysis-targeting chimeras: a new frontier for drug discovery. Front Chem. 2021;9(70): 639176.
Reynders M, Trauner D. Optical control of targeted protein degradation. Cell Chem Biol. 2021;28(7):969–86.
Liu J, Peng Y, Wei W. Light-controllable PROTACs for temporospatial control of protein degradation. Front Cell Dev Biol. 2021;9(1777): 678077.
Wu P, Manna D. Optochemical control of protein degradation. ChemBioChem. 2020;21(16):2250–2.
Kounde CS, Tate EW. Photoactive bifunctional degraders: precision tools to regulate protein stability. J Med Chem. 2020;63(24):5483–93.
Yu H, Li J, Wu D, Qiu Z, Zhang Y. Chemistry and biological applications of photo-labile organic molecules. Chem Soc Rev. 2010;39(2):464–73.
Xue G, Wang K, Zhou D, Zhong H, Pan Z. Light-induced protein degradation with photocaged PROTACs. J Am Chem Soc. 2019;141(46):18370–4.
Naro Y, Darrah K, Deiters A. Optical control of small molecule-induced protein degradation. J Am Chem Soc. 2020;142(5):2193–7.
Kounde CS, Shchepinova MM, Saunders CN, Muelbaier M, Rackham MD, Harling JD, et al. A caged E3 ligase ligand for PROTAC-mediated protein degradation with light. Chem Commun. 2020;56(41):5532–5.
Liu J, Chen H, Ma L, He Z, Wang D, Liu Y, et al. Light-induced control of protein destruction by opto-PROTAC. Sci Adv. 2020;6(8):eaay5154.
Li Z, Ma S, Yang X, Zhang L, Liang D, Dong G, et al. Development of photocontrolled BRD4 PROTACs for tongue squamous cell carcinoma (TSCC). Eur J Med Chem. 2021;222: 113608.
Harris JD, Moran MJ, Aprahamian I. New molecular switch architectures. Proc Natl Acad Sci USA. 2018;115(38):9414–22.
Pfaff P, Samarasinghe KTG, Crews CM, Carreira EM. Reversible spatiotemporal control of induced protein degradation by bistable photoPROTACs. ACS Cent Sci. 2019;5(10):1682–90.
Reynders M, Matsuura BS, Bérouti M, Simoneschi D, Marzio A, Pagano M, et al. PHOTACs enable optical control of protein degradation. Sci Adv. 2020;6(8):eaay5064.
Jin YH, Lu MC, Wang Y, Shan WX, Wang XY, You QD, et al. Azo-PROTAC: novel light-controlled small-molecule tool for protein knockdown. J Med Chem. 2020;63(9):4644–54.
Zhang C, Pu K. Molecular and nanoengineering approaches towards activatable cancer immunotherapy. Chem Soc Rev. 2020;49(13):4234–53.
Zhang P, Gao D, An K, Shen Q, Wang C, Zhang Y, et al. A programmable polymer library that enables the construction of stimuli-responsive nanocarriers containing logic gates. Nat Chem. 2020;12(4):381–90.
Xu X, Lu H, Lee R. Near infrared light triggered photo/immuno-therapy toward cancers. Front Bioeng Biotechnol. 2020;8:488.
Geng J, Zhang Y, Gao Q, Neumann K, Dong H, Porter H, et al. Switching on prodrugs using radiotherapy. Nat Chem. 2021;13(8):805–10.
Huo S, Zhao P, Shi Z, Zou M, Yang X, Warszawik E, et al. Mechanochemical bond scission for the activation of drugs. Nat Chem. 2021;13(2):131–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this article.
Conflicts of Interest/Competing Interests
The authors have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All the authors have approved and agreed to publish this manuscript.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Authors’ Contributions
L. Chen, X. Wan, and X. Shan wrote the manuscript and prepared the figures with equal contributions. W. Zha proofread the manuscript. R. Fan raised the concept, conducted the literature review, and wrote and revised the manuscript and figures. All authors approved the final manuscript.
Rights and permissions
About this article
Cite this article
Chen, L., Wan, X., Shan, X. et al. Smart PROTACs Enable Controllable Protein Degradation for Precision Cancer Therapy. Mol Diagn Ther 26, 283–291 (2022). https://doi.org/10.1007/s40291-022-00586-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-022-00586-2