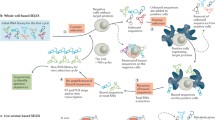
Abstract
Aptamers are synthetic DNA or RNA oligonucleotide ligands with great potential for therapeutic applications. A vast number of disease-related targets have been used to identify agonistic, antagonistic, or inhibitory aptamers, or aptamer-based targeting ligands. However, only a few aptamers have reached late-stage clinical trials so far and the commercial infrastructure is still far behind that of other therapeutic agents such as monoclonal antibodies. The desirable properties of aptamers such as selectivity, chemical flexibility, or cost-efficiency are faced by challenges, including a short half-life in vivo, immunogenicity, and entrapment in cellular organelles. Aptamer research is still in an early stage, and a deeper understanding of their structure, target interactions, and pharmacokinetics is necessary to catch up to the clinical market. In this review, we will discuss the benefits and limitations in the development of therapeutic aptamers, as well as the advances and future directions of aptamer research. The progress towards effective therapies seems to be slow, but it has not stopped and the best is yet to come.

Similar content being viewed by others
References
Hermann T, Patel DJ. Adaptive recognition by nucleic acid aptamers. Science. 2000;287(5454):820–5.
Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346(6287):818–22. https://doi.org/10.1038/346818a0.
Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249(4968):505–10.
Gilboa E, McNamara J 2nd, Pastor F. Use of oligonucleotide aptamer ligands to modulate the function of immune receptors. Clin Cancer Res. 2013;19(5):1054–62. https://doi.org/10.1158/1078-0432.CCR-12-2067.
Zhou J, Rossi JJ. Cell-type-specific, aptamer-functionalized agents for targeted disease therapy. Mol Ther Nucleic Acids. 2014;3:e169. https://doi.org/10.1038/mtna.2014.21.
Keefe AD, Pai S, Ellington A. Aptamers as therapeutics. Nat Rev Drug Discov. 2010;9(7):537–50. https://doi.org/10.1038/nrd3141.
Bock LC, Griffin LC, Latham JA, Vermaas EH, Toole JJ. Selection of single-stranded DNA molecules that bind and inhibit human thrombin. Nature. 1992;355(6360):564–6. https://doi.org/10.1038/355564a0.
Kubik MF, Stephens AW, Schneider D, Marlar RA, Tasset D. High-affinity RNA ligands to human alpha-thrombin. Nucleic Acids Res. 1994;22(13):2619–26.
Bompiani KM, Monroe DM, Church FC, Sullenger BA. A high affinity, antidote-controllable prothrombin and thrombin-binding RNA aptamer inhibits thrombin generation and thrombin activity. J Thromb Haemost. 2012;10(5):870–80. https://doi.org/10.1111/j.1538-7836.2012.04679.x.
Tasset DM, Kubik MF, Steiner W. Oligonucleotide inhibitors of human thrombin that bind distinct epitopes. J Mol Biol. 1997;272(5):688–98. https://doi.org/10.1006/jmbi.1997.1275.
White R, Rusconi C, Scardino E, Wolberg A, Lawson J, Hoffman M, et al. Generation of species cross-reactive aptamers using “toggle” SELEX. Mol Ther. 2001;4(6):567–73. https://doi.org/10.1006/mthe.2001.0495.
Pagratis NC, Bell C, Chang YF, Jennings S, Fitzwater T, Jellinek D, et al. Potent 2’-amino-, and 2’-fluoro-2’-deoxyribonucleotide RNA inhibitors of keratinocyte growth factor. Nat Biotechnol. 1997;15(1):68–73. https://doi.org/10.1038/nbt0197-68.
Binkley J, Allen P, Brown DM, Green L, Tuerk C, Gold L. RNA ligands to human nerve growth factor. Nucleic Acids Res. 1995;23(16):3198–205.
Ng EW, Shima DT, Calias P, Cunningham ET Jr, Guyer DR, Adamis AP. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov. 2006;5(2):123–32. https://doi.org/10.1038/nrd1955.
Padilla R, Sousa R. Efficient synthesis of nucleic acids heavily modified with non-canonical ribose 2’-groups using a mutantT7 RNA polymerase (RNAP). Nucleic Acids Res. 1999;27(6):1561–3.
Lee Y, Urban JH, Xu L, Sullenger BA, Lee J. 2’Fluoro modification differentially modulates the ability of RNAs to activate pattern recognition receptors. Nucleic Acid Ther. 2016;26(3):173–82. https://doi.org/10.1089/nat.2015.0575.
Biesecker G, Dihel L, Enney K, Bendele RA. Derivation of RNA aptamer inhibitors of human complement C5. Immunopharmacology. 1999;42(1–3):219–30.
Rusconi CP, Scardino E, Layzer J, Pitoc GA, Ortel TL, Monroe D, et al. RNA aptamers as reversible antagonists of coagulation factor IXa. Nature. 2002;419(6902):90–4. https://doi.org/10.1038/nature00963.
Santulli-Marotto S, Nair SK, Rusconi C, Sullenger B, Gilboa E. Multivalent RNA aptamers that inhibit CTLA-4 and enhance tumor immunity. Cancer Res. 2003;63(21):7483–9.
Fülle L, Steiner N, Funke M, Gondorf F, Pfeiffer F, Siegl J, et al. RNA aptamers recognizing murine CCL17 Inhibit t cell chemotaxis and reduce contact hypersensitivity in vivo. Mol Ther. 2018;26(1):95–104. https://doi.org/10.1016/j.ymthe.2017.10.005.
Maier KE, Levy M. From selection hits to clinical leads: progress in aptamer discovery. Mol Ther Methods Clin Dev. 2016;5:16014. https://doi.org/10.1038/mtm.2016.14.
Domenyuk V, Gatalica Z, Santhanam R, Wei X, Stark A, Kennedy P, et al. Poly-ligand profiling differentiates trastuzumab-treated breast cancer patients according to their outcomes. Nat Commun. 2018;9(1):1219. https://doi.org/10.1038/s41467-018-03631-z.
Mi J, Liu Y, Rabbani ZN, Yang Z, Urban JH, Sullenger BA, et al. In vivo selection of tumor-targeting RNA motifs. Nat Chem Biol. 2010;6(1):22–4. https://doi.org/10.1038/nchembio.277.
Pereira RL, Nascimento IC, Santos AP, Ogusuku IEY, Lameu C, Mayer G, et al. Aptamers: novelty tools for cancer biology. Oncotarget. 2018;9(42):26934–53. https://doi.org/10.18632/oncotarget.25260.
McNamara JO, Kolonias D, Pastor F, Mittler RS, Chen L, Giangrande PH, et al. Multivalent 4-1BB binding aptamers costimulate CD8+ T cells and inhibit tumor growth in mice. J Clin Investig. 2008;118(1):376–86. https://doi.org/10.1172/JCI33365.
Prodeus A, Abdul-Wahid A, Fischer NW, Huang EH, Cydzik M, Gariepy J. Targeting the PD-1/PD-L1 immune evasion axis with DNA aptamers as a novel therapeutic strategy for the treatment of disseminated cancers. Mol Ther Nucleic Acids. 2015;4:e237. https://doi.org/10.1038/mtna.2015.11.
Lai WY, Huang BT, Wang JW, Lin PY, Yang PC. A novel PD-L1-targeting antagonistic DNA aptamer with antitumor effects. Mol Ther Nucleic Acids. 2016;5(12):e397. https://doi.org/10.1038/mtna.2016.102.
Wang H, Lam CH, Li X, West DL, Yang X. Selection of PD1/PD-L1 X-aptamers. Biochimie. 2018;145:125–30. https://doi.org/10.1016/j.biochi.2017.09.006.
Huang BT, Lai WY, Chang YC, Wang JW, Yeh SD, Lin EP, et al. A CTLA-4 antagonizing DNA aptamer with antitumor effect. Mol Ther Nucleic Acids. 2017;8:520–8. https://doi.org/10.1016/j.omtn.2017.08.006.
Lupold SE, Hicke BJ, Lin Y, Coffey DS. Identification and characterization of nuclease-stabilized RNA molecules that bind human prostate cancer cells via the prostate-specific membrane antigen. Cancer Res. 2002;62(14):4029–33.
Cerchia L, Esposito CL, Camorani S, Rienzo A, Stasio L, Insabato L, et al. Targeting Axl with an high-affinity inhibitory aptamer. Mol Ther. 2012;20(12):2291–303. https://doi.org/10.1038/mt.2012.163.
Lupold SE. Aptamers and apple pies: a mini-review of PSMA aptamers and lessons from Donald S. Coffey. Am J Clin Exp Urol. 2018;6(2):78–86.
Esposito CL, Cerchia L, Catuogno S, De Vita G, Dassie JP, Santamaria G, et al. Multifunctional aptamer-miRNA conjugates for targeted cancer therapy. Mol Ther. 2014;22(6):1151–63. https://doi.org/10.1038/mt.2014.5.
Iaboni M, Russo V, Fontanella R, Roscigno G, Fiore D, Donnarumma E, et al. Aptamer-miRNA-212 conjugate sensitizes NSCLC cells to TRAIL. Mol Ther Nucleic Acids. 2016;5:e289. https://doi.org/10.1038/mtna.2016.5.
Russo V, Paciocco A, Affinito A, Roscigno G, Fiore D, Palma F, et al. Aptamer-miR-34c conjugate affects cell proliferation of non-small-cell lung cancer cells. Mol Ther Nucleic Acids. 2018;13:334–46. https://doi.org/10.1016/j.omtn.2018.09.016.
Esposito CL, Nuzzo S, Kumar SA, Rienzo A, Lawrence CL, Pallini R, et al. A combined microRNA-based targeted therapeutic approach to eradicate glioblastoma stem-like cells. J Control Release. 2016;238:43–57. https://doi.org/10.1016/j.jconrel.2016.07.032.
Green LS, Jellinek D, Jenison R, Ostman A, Heldin CH, Janjic N. Inhibitory DNA ligands to platelet-derived growth factor B-chain. Biochemistry. 1996;35(45):14413–24. https://doi.org/10.1021/bi961544+.
Dunn EN, Hariprasad SM, Sheth VS. An overview of the Fovista and Rinucumab trials and the fate of anti-PDGF medications. Ophthalmic Surg Lasers Imaging Retina. 2017;48(2):100–4. https://doi.org/10.3928/23258160-20170130-02.
Cohen MG, Purdy DA, Rossi JS, Grinfeld LR, Myles SK, Aberle LH, et al. First clinical application of an actively reversible direct factor IXa inhibitor as an anticoagulation strategy in patients undergoing percutaneous coronary intervention. Circulation. 2010;122(6):614–22. https://doi.org/10.1161/CIRCULATIONAHA.109.927756.
Ganson NJ, Povsic TJ, Sullenger BA, Alexander JH, Zelenkofske SL, Sailstad JM, et al. Pre-existing anti-polyethylene glycol antibody linked to first-exposure allergic reactions to pegnivacogin, a PEGylated RNA aptamer. J Allergy Clin Immunol. 2016;137(5):1610 e7–1613 e7. https://doi.org/10.1016/j.jaci.2015.10.034.
Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005;333(2):328–35. https://doi.org/10.1016/j.bbrc.2005.05.132.
Lowe J, Araujo J, Yang J, Reich M, Oldendorp A, Shiu V, et al. Ranibizumab inhibits multiple forms of biologically active vascular endothelial growth factor in vitro and in vivo. Exp Eye Res. 2007;85(4):425–30. https://doi.org/10.1016/j.exer.2007.05.008.
Semeraro F, Morescalchi F, Duse S, Parmeggiani F, Gambicorti E, Costagliola C. Aflibercept in wet AMD: specific role and optimal use. Drug Des Devel Ther. 2013;7:711–22. https://doi.org/10.2147/DDDT.S40215.
D’Amore PA. Vascular endothelial cell growth factor-a: not just for endothelial cells anymore. Am J Pathol. 2007;171(1):14–8. https://doi.org/10.2353/ajpath.2007.070385.
Drolet DW, Green LS, Gold L, Janjic N. Fit for the eye: aptamers in ocular disorders. Nucleic Acid Ther. 2016;26(3):127–46. https://doi.org/10.1089/nat.2015.0573.
Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. MAbs. 2015;7(1):9–14. https://doi.org/10.4161/19420862.2015.989042.
Hernandez FJ, Kalra N, Wengel J, Vester B. Aptamers as a model for functional evaluation of LNA and 2’-amino LNA. Bioorg Med Chem Lett. 2009;19(23):6585–7. https://doi.org/10.1016/j.bmcl.2009.10.039.
Pinheiro VB, Taylor AI, Cozens C, Abramov M, Renders M, Zhang S, et al. Synthetic genetic polymers capable of heredity and evolution. Science. 2012;336(6079):341–4. https://doi.org/10.1126/science.1217622.
Green LS, Jellinek D, Bell C, Beebe LA, Feistner BD, Gill SC, et al. Nuclease-resistant nucleic acid ligands to vascular permeability factor/vascular endothelial growth factor. Chem Biol. 1995;2(10):683–95.
Dass CR, Saravolac EG, Li Y, Sun LQ. Cellular uptake, distribution, and stability of 10–23 deoxyribozymes. Antisense Nucleic Acid Drug Dev. 2002;12(5):289–99. https://doi.org/10.1089/108729002761381276.
Lauridsen LH, Rothnagel JA, Veedu RN. Enzymatic recognition of 2’-modified ribonucleoside 5’-triphosphates: towards the evolution of versatile aptamers. Chembiochem. 2012;13(1):19–25. https://doi.org/10.1002/cbic.201100648.
Loakes D, Holliger P. Polymerase engineering: towards the encoded synthesis of unnatural biopolymers. Chem Commun (Camb). 2009;31:4619–31. https://doi.org/10.1039/b903307f.
Meyer AJ, Garry DJ, Hall B, Byrom MM, McDonald HG, Yang X, et al. Transcription yield of fully 2’-modified RNA can be increased by the addition of thermostabilizing mutations to T7 RNA polymerase mutants. Nucleic Acids Res. 2015;43(15):7480–8. https://doi.org/10.1093/nar/gkv734.
Vater A, Klussmann S. Turning mirror-image oligonucleotides into drugs: the evolution of Spiegelmer((R)) therapeutics. Drug Discov Today. 2015;20(1):147–55. https://doi.org/10.1016/j.drudis.2014.09.004.
Nolte A, Klussmann S, Bald R, Erdmann VA, Furste JP. Mirror-design of l-oligonucleotide ligands binding to l-arginine. Nat Biotechnol. 1996;14(9):1116–9. https://doi.org/10.1038/nbt0996-1116.
Klussmann S, Nolte A, Bald R, Erdmann VA, Furste JP. Mirror-image RNA that binds d-adenosine. Nat Biotechnol. 1996;14(9):1112–5. https://doi.org/10.1038/nbt0996-1112.
Hoellenriegel J, Zboralski D, Maasch C, Rosin NY, Wierda WG, Keating MJ, et al. The Spiegelmer NOX-A12, a novel CXCL12 inhibitor, interferes with chronic lymphocytic leukemia cell motility and causes chemosensitization. Blood. 2014;123(7):1032–9. https://doi.org/10.1182/blood-2013-03-493924.
Ninichuk V, Clauss S, Kulkarni O, Schmid H, Segerer S, Radomska E, et al. Late onset of Ccl2 blockade with the Spiegelmer mNOX-E36-3’PEG prevents glomerulosclerosis and improves glomerular filtration rate in db/db mice. Am J Pathol. 2008;172(3):628–37. https://doi.org/10.2353/ajpath.2008.070601.
Schwoebel F, van Eijk LT, Zboralski D, Sell S, Buchner K, Maasch C, et al. The effects of the anti-hepcidin Spiegelmer NOX-H94 on inflammation-induced anemia in cynomolgus monkeys. Blood. 2013;121(12):2311–5. https://doi.org/10.1182/blood-2012-09-456756.
Healy JM, Lewis SD, Kurz M, Boomer RM, Thompson KM, Wilson C, et al. Pharmacokinetics and biodistribution of novel aptamer compositions. Pharm Res. 2004;21(12):2234–46.
Lee CH, Lee SH, Kim JH, Noh YH, Noh GJ, Lee SW. Pharmacokinetics of a cholesterol-conjugated aptamer against the hepatitis C virus (HCV) NS5B protein. Mol Ther Nucleic Acids. 2015;4:e254. https://doi.org/10.1038/mtna.2015.30.
Zhang P, Sun F, Liu S, Jiang S. Anti-PEG antibodies in the clinic: current issues and beyond PEGylation. J Control Release. 2016;244(Pt B):184–93. https://doi.org/10.1016/j.jconrel.2016.06.040.
Bruno JG. Potential inherent stimulation of the innate immune system by nucleic acid aptamers and possible corrective approaches. Pharmaceuticals (Basel). 2018. https://doi.org/10.3390/ph11030062.
Civit L, Taghdisi SM, Jonczyk A, Hassel SK, Grober C, Blank M, et al. Systematic evaluation of cell-SELEX enriched aptamers binding to breast cancer cells. Biochimie. 2018;145:53–62. https://doi.org/10.1016/j.biochi.2017.10.007.
Avci-Adali M, Steinle H, Michel T, Schlensak C, Wendel HP. Potential capacity of aptamers to trigger immune activation in human blood. PLoS One. 2013;8(7):e68810. https://doi.org/10.1371/journal.pone.0068810.
D’Amico DJ, Masonson HN, Patel M, Adamis AP, Cunningham ET Jr, Guyer DR, et al. Pegaptanib sodium for neovascular age-related macular degeneration: two-year safety results of the two prospective, multicenter, controlled clinical trials. Ophthalmology. 2006;113(6):992 e6–1001 e6. https://doi.org/10.1016/j.ophtha.2006.02.027.
Farman CA, Kornbrust DJ. Oligodeoxynucleotide studies in primates: antisense and immune stimulatory indications. Toxicol Pathol. 2003;31(Suppl):119–22. https://doi.org/10.1080/01926230390174995.
Goebl N, Berridge B, Wroblewski VJ, Brown-Augsburger PL. Development of a sensitive and specific in situ hybridization technique for the cellular localization of antisense oligodeoxynucleotide drugs in tissue sections. Toxicol Pathol. 2007;35(4):541–8. https://doi.org/10.1080/01926230701338958.
Henry SP, Zuckerman JE, Rojko J, Hall WC, Harman RJ, Kitchen D, et al. Toxicological properties of several novel oligonucleotide analogs in mice. Anticancer Drug Des. 1997;12(1):1–14.
Paul A, Avci-Adali M, Neumann B, Guo K, Straub A, Dietz K, et al. Aptamers influence the hemostatic system by activating the intrinsic coagulation pathway in an in vitro Chandler-Loop model. Clin Appl Thromb Hemost. 2010;16(2):161–9. https://doi.org/10.1177/1076029608329580.
Gansler J, Jaax M, Leiting S, Appel B, Greinacher A, Fischer S, et al. Structural requirements for the procoagulant activity of nucleic acids. PLoS One. 2012;7(11):e50399. https://doi.org/10.1371/journal.pone.0050399.
Perschbacher K, Smestad JA, Peters JP, Standiford MM, Denic A, Wootla B, et al. Quantitative PCR analysis of DNA aptamer pharmacokinetics in mice. Nucleic Acid Ther. 2015;25(1):11–9. https://doi.org/10.1089/nat.2014.0515.
Thiel WH, Thiel KW, Flenker KS, Bair T, Dupuy AJ, McNamara JO 2nd, et al. Cell-internalization SELEX: method for identifying cell-internalizing RNA aptamers for delivering siRNAs to target cells. Methods Mol Biol. 2015;1218:187–99. https://doi.org/10.1007/978-1-4939-1538-5_11.
Yan A, Levy M. Cell internalization SELEX: in vitro selection for molecules that internalize into cells. Methods Mol Biol. 2014;1103:241–65. https://doi.org/10.1007/978-1-62703-730-3_18.
Tawiah KD, Porciani D, Burke DH. Toward the selection of cell targeting aptamers with extended biological functionalities to facilitate endosomal escape of cargoes. Biomedicines. 2017. https://doi.org/10.3390/biomedicines5030051.
Mayer G, Pofahl M, Schöler KMU, Haßel S. Cell-specific aptamers for nano-medical applications. In: Kjems J, Ferapontova E, Gothelf K, editors. Nucleic acid nanotechnology. Berlin: Springer; 2014.
Dowdy SF. Overcoming cellular barriers for RNA therapeutics. Nat Biotechnol. 2017;35(3):222–9. https://doi.org/10.1038/nbt.3802.
Liu HY, Gao X. A universal protein tag for delivery of SiRNA-aptamer chimeras. Sci Rep. 2013;3:3129. https://doi.org/10.1038/srep03129.
Nossal GJ, Lederberg J. Antibody production by single cells. Nature. 1958;181(4620):1419–20.
Ribatti D. Edelman’s view on the discovery of antibodies. Immunol Lett. 2015;164(2):72–5. https://doi.org/10.1016/j.imlet.2015.02.005.
Hey A. History and practice: antibodies in infectious diseases. Microbiol Spectr. 2015;3(2):AID-0026-2014. https://doi.org/10.1128/microbiolspec.aid-0026-2014.
Llewelyn MB, Hawkins RE, Russell SJ. Discovery of antibodies. BMJ. 1992;305(6864):1269–72.
Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495–7.
Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;302(5909):575–81.
Buss NA, Henderson SJ, McFarlane M, Shenton JM, de Haan L. Monoclonal antibody therapeutics: history and future. Curr Opin Pharmacol. 2012;12(5):615–22. https://doi.org/10.1016/j.coph.2012.08.001.
Robertson DL, Joyce GF. Selection in vitro of an RNA enzyme that specifically cleaves single-stranded DNA. Nature. 1990;344(6265):467–8. https://doi.org/10.1038/344467a0.
Bates PJ, Laber DA, Miller DM, Thomas SD, Trent JO. Discovery and development of the G-rich oligonucleotide AS1411 as a novel treatment for cancer. Exp Mol Pathol. 2009;86(3):151–64. https://doi.org/10.1016/j.yexmp.2009.01.004.
Mongelard F, Bouvet P. AS-1411, a guanosine-rich oligonucleotide aptamer targeting nucleolin for the potential treatment of cancer, including acute myeloid leukemia. Curr Opin Mol Ther. 2010;12(1):107–14.
Reyes-Reyes EM, Teng Y, Bates PJ. A new paradigm for aptamer therapeutic AS1411 action: uptake by macropinocytosis and its stimulation by a nucleolin-dependent mechanism. Cancer Res. 2010;70(21):8617–29. https://doi.org/10.1158/0008-5472.CAN-10-0920.
Baker M. Reproducibility crisis: blame it on the antibodies. Nature. 2015;521(7552):274–6. https://doi.org/10.1038/521274a.
Bradbury A, Pluckthun A. Reproducibility: standardize antibodies used in research. Nature. 2015;518(7537):27–9. https://doi.org/10.1038/518027a.
Stein CA, Castanotto D. FDA-approved oligonucleotide therapies in 2017. Mol Ther. 2017;25(5):1069–75. https://doi.org/10.1016/j.ymthe.2017.03.023.
Smith CIE, Zain R. Therapeutic oligonucleotides: state of the art. Annu Rev Pharmacol Toxicol. 2019;59:605–30. https://doi.org/10.1146/annurev-pharmtox-010818-021050.
Diener JL, Daniel Lagasse HA, Duerschmied D, Merhi Y, Tanguay JF, Hutabarat R, et al. Inhibition of von Willebrand factor-mediated platelet activation and thrombosis by the anti-von Willebrand factor A1-domain aptamer ARC1779. J Thromb Haemost. 2009;7(7):1155–62. https://doi.org/10.1111/j.1538-7836.2009.03459.x.
Troisi R, Napolitano V, Spiridonova V, Russo Krauss I, Sica F. Several structural motifs cooperate in determining the highly effective anti-thrombin activity of NU172 aptamer. Nucleic Acids Res. 2018;46(22):12177–85. https://doi.org/10.1093/nar/gky990.
Waters EK, Genga RM, Schwartz MC, Nelson JA, Schaub RG, Olson KA, et al. Aptamer ARC19499 mediates a procoagulant hemostatic effect by inhibiting tissue factor pathway inhibitor. Blood. 2011;117(20):5514–22. https://doi.org/10.1182/blood-2010-10-311936.
Kulkarni O, Pawar RD, Purschke W, Eulberg D, Selve N, Buchner K, et al. Spiegelmer inhibition of CCL2/MCP-1 ameliorates lupus nephritis in MRL-(Fas)lpr mice. J Am Soc Nephrol. 2007;18(8):2350–8. https://doi.org/10.1681/ASN.2006121348.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
No external funding was used in the preparation of this review.
Conflict of interest
Silvana K. Haßel and Günter Mayer have no conflicts of interest that are directly relevant to the content of this review.
Rights and permissions
About this article
Cite this article
Haßel, S.K., Mayer, G. Aptamers as Therapeutic Agents: Has the Initial Euphoria Subsided?. Mol Diagn Ther 23, 301–309 (2019). https://doi.org/10.1007/s40291-019-00400-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-019-00400-6