Abstract
Background
Hepatitis C infection is a major health problem worldwide, especially in Egypt. The high prevalence of mixed cryoglobulinemia (MC) in hepatitis C patients leads to the assumption that there is a direct link between hepatitis C virus (HCV) and cryoglobulinemia. Host genetic factors could be a contributing factor. B cell-activating factor (BAFF) is a tumor necrosis factor (TNF) family member, which has an essential role in B lymphocyte development and survival. The aim of the present work was to study the possible association between the BAFF −871C/T promoter polymorphism and HCV-related MC in a cohort of Egyptian patients.
Methods
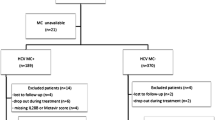
The study was conducted in 120 HCV patients classified into two groups: group I (60 HCV patients with MC) and group II (60 HCV patients without MC), with 60 age- and sex-matched healthy control subjects. BAFF −871C/T genotyping was performed in all subjects by polymerase chain reaction (PCR) with restriction fragment length polymorphism analysis.
Results
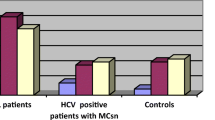
The prevalence of the BAFF −871TT genotype was significantly increased in HCV patients compared with the control group (P = 0.036). The BAFF TT genotype was also significantly more prevalent in group I (HCV–MC patients) than in group II (HCV patients without MC) [P < 0.001].
Conclusion
A significant association was found between the BAFF −871C/T promoter polymorphism and MC, which may indicate that BAFF could be a potential therapeutic target in HCV–MC.
Similar content being viewed by others
References
Charles ED, Dustin LB. Hepatitis C virus-induced cryoglobulinemia. Kidney Int. 2009;76(8):818–24.
Strickland GT, Elhefni H, Salman T, Waked I, Abdel-Hamid M, Mikhail NN, et al. Role of hepatitis C infection in chronic liver disease in Egypt. Am J Trop Med Hyg. 2002;67(4):436–42.
Strickland GT. Liver disease in Egypt: hepatitis C superseded schistosomiasis as a result of iatrogenic and biological factors. Hepatology. 2006;43(5):915–22.
Zignego AL, Gragnani L, Giannini C, Laffi G. The hepatitis C virus infection as a systemic disease. Intern Emerg Med. 2012;7(3):201–8.
Dammacco F, Sansonno D, Piccoli C, et al. The cryoglobulins: an overview. Eur J Clin Invest. 2001;31:628–38.
Morra E. Cryoglobulinemia. Hematol Am Soc Hematol Educ Program. 2005;1:368–72.
Kallemuchikkal U, Gorevic PD. Evaluation of cryoglobulins. Arch Pathol Lab Med. 1999;123:119–25.
Meltzer M, Franklin EC, Elias K, McCluskey RT, Cooper N. Cryoglobulinemia—a clinical and laboratory study. II. Cryoglobulins with rheumatoid factor activity. Am J Med. 1966;40(6):837–56.
Zignego AL, Gragnani L, Piluso A, Sebastiani M, Giuggioli D, Fallahi P, Antonelli A, Ferri C. Virus-driven autoimmunity and lymphoproliferation: the example of HCV infection. Expert Rev Clin Immunol. 2015;11(1):15–31.
Sene D, Ghillani-Dalbin P, Thibault V, et al. Long term course of mixed cryoglobulinemia in patients infected with hepatitis C virus. J Rheumatol. 2004;31:2199–206.
Ramos-Casals M, Robles A, Brito-Zerón P, et al. Life-threatening cryoglobulinemia: clinical and immunological characterization of 29 cases. Semin Arthr Rheum. 2006;36:189–96.
Ferri C, Giuggioli D, Cazzato M, Sebastiani M, Mascia MT, Zignego AL. HCV-related cryoglobulinemic vasculitis: an update on its etiopathogenesis and therapeutic strategies. Clin Exp Rheumatol. 2003;21(6 Suppl 32):78–84.
Zignego AL, Giannelli F, Marrocchi ME, Mazzocca A, Ferri C, Giannini C, Monti M, Caini P, Villa GL, Laffi G, Gentilini P. T(14;18) translocation in chronic hepatitis C virus infection. Hepatology. 2000;31(2):474–9.
Ni J, Hembrador E, Di Bisceglie AM, et al. Accumulation of B lymphocytes with a naive, resting phenotype in a subset of hepatitis C patients. J Immunol. 2003;170:3429–39.
Mackay F, Woodcock SA, Lawton P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–710.
Avery DT, Kalled SL, Ellyard JI, et al. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J Clin Invest. 2003;112:286–97.
Lesley R, Xu Y, Kalled SL, et al. Reduced competitiveness of auto antigen engaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441–53.
Thien M, Phan TG, Gardam S, et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–98.
Cheema GS, Roschke V, Hilbert DM, Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthr Rheum. 2001;44:1313–9.
Ittah M, Miceli-Richard C, Eric Gottenberg J, et al. B cell-activating factor of the tumor necrosis factor family (BAFF) is expressed under stimulation by interferon in salivary gland epithelial cells in primary Sjogren’s syndrome. Arthr Res Ther. 2006;8:51.
Giannini C, Gragnani L, Piluso A, Caini P, Petrarca A, Monti M, Laffi G, Zignego AL. Can BAFF promoter polymorphism be a predisposing condition for HCV-related mixed cryoglobulinemia? Blood. 2008;112(10):4353–4.
Sargur R, White P, Egner W. Cryoglobulin evaluation: best practice? Ann Clin Biochem. 2010;47:8–16.
Lissoir B, Wallemacq P, Maisin D. Serum protein electrophoresis: comparison of capillary zone electrophoresis Capillarys (Sebia) and agarose gel electrophoresis Hydrasys (Sebia). Ann Biol Clin. 2003;61(5):557–62.
Novak AJ, Grote DM, Ziesmer SC, Kline MP, Manske MK, Slager S, et al. Elevated serum B-lymphocyte stimulator levels in patients with familial lymphoproliferative disorders. J Clin Oncol. 2006;24:983–7.
Gragnani L, Fognani E, Piluso A, Zignego AL. Hepatitis C virus-related mixed cryoglobulinemia: is genetics to blame? World J Gastroenterol. 2013;19(47):8910–5.
Bianchettin G, Bonaccini C, Oliva R, Tramontano A, Cividini A, Casato M, et al. Analysis of hepatitis C virus hypervariable region 1 sequence from cryoglobulinemic patients and associated controls. J Virol. 2007;81:4564–71.
De Vita S, Quartuccio L, Fabris M. Hepatitis C virus infection, mixed cryoglobulinemia and BLyS upregulation: targeting the infectious trigger, the autoimmune response, or both? Autoimmun Rev. 2008;8:95–9.
Shahin AA, Zayed HS, Elshazly MS. Evaluation of the liver condition in chronic hepatitis C virus patients with and without vasculitis. Egypt Rheumatol. 2014;36(4):187–93.
Donada C, Crucitti A, Donadon V, Tommasi L, Zanette G, Crovatto M, et al. Systemic manifestations and liver disease in patients with chronic hepatitis C and type II or III mixed cryoglobulinaemia. J Viral Hepat. 1998;5:179–85.
Kayali Z, Buckwold VE, Zimmerman B, Schmidt WN. Hepatitis C, cryoglobulinemia, and cirrhosis: a meta-analysis. Hepatology. 2002;36:978–85.
Monti G, Galli M, Invernizzi F, Pioltelli P, Saccardo F, Monteverde A, et al. Cryoglobulinaemias: a multi-centre study of the early clinical and laboratory manifestations of primary and secondary disease. GISC. Italian Group for the Study of Cryoglobulinaemias. QJM. 1995;88:115–26.
Cacoub P, Poynard T, Ghillani P, Charlotte F, Olivi M, Piette JC, et al. Extrahepatic manifestations of chronic hepatitis C. Multivirc group. Multidepartment Virus C. Arthr Rheum. 1999;42:2204–12.
Ferri C. Mixed cryoglobulinemia. Orphanet J Rare Dis. 2008;3:25.
Saadoun D, Asselah T, Resche-Rigon M, Charlotte F, Bedossa P, Valla D, et al. Cryoglobulinemia is associated with steatosis and fibrosis in chronic hepatitis C. Hepatology. 2006;43:1337–45.
Cresta P, Musset L, Cacoub P, Frangeul L, Vitour D, Poynard T, et al. Response to interferon alpha treatment and disappearance of cryoglobulinaemia in patients infected by hepatitis C virus. Gut. 1999;45:122–8.
Agnello V, Chung RT, Kaplan LM. A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med. 1992;327:1490–5.
Terrier B, Semoun O, Saadoun D, Sène D, Resche-Rigon M, Cacoub P. Prognostic factors in patients with hepatitis C virus infection and systemic vasculitis. Arthr Rheum. 2011;63:1748–75.
Saadoun D, Terrier B, Semoun O, Sene D, Maisonobe T, Musset L, et al. Hepatitis C virus-associated polyarteritis nodosa. Arthr Care Res (Hoboken). 2011;63:427–35.
Gragnani L, Piluso A, Giannini C, Caini P, Fognani E, et al. Genetic determinants in hepatitis C virus-associated mixed cryoglobulinemia: role of polymorphic variants of BAFF promoter and Fcγ receptors. Arthr Rheum. 2011;63(5):1446–51.
Groom J, Kalled SL, Cutler AH, Olson C, Woodcock SA, Schneider P, Tschopp J, Cachero TG, Batten M, Wheway J, Mauri D, Cavill D, Gordon TP, Mackay CR, Mackay F. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren’s syndrome. J Clin Investig. 2002;109:59–68.
Jonsson MV, Szodoray P, Jellestad S, Jonsson R, Skarstein K. Association between circulating levels of the novel TNF family members APRIL and BAFF and lymphoid organization in primary Sjogren’s syndrome. J Clin Immunol. 2005;25:189–201.
Sene D, Ghillani-Dalbin P, Thibault V, Guis L, Musset L, Duhaut P, Poynard T, Piette JC, Cacoub P. Longterm course of mixed cryoglobulinemia in patients infected with hepatitis C virus. J Rheumatol. 2004;31(11):2199–206.
Toubi E, Gordon S, Kessel A, et al. Elevated serum B-lymphocyte activating factor (BAFF) in chronic hepatitis C virus infection: association with autoimmunity. J Autoimmun. 2006;27:134–9.
Fabris M, Quartuccio L, Sacco S, et al. B-lymphocyte stimulator (BLyS) upregulation in mixed cryoglobulinaemia syndrome and hepatitis-C virus infection. Rheumatology. 2007;46:37–43.
Tarantino G, Marco VD, Petta A, et al. Serum BLyS/BAFF predicts the outcome of acute hepatitis C virus infection. J Viral Hepat. 2009;16(6):397–405.
Vincent FB, Morand EF, Mackay F. BAFF and innate immunity: new therapeutic targets for systemic lupus erythematosus. Immunol Cell Biol. 2012;90(3):293–303.
Acknowledgments and disclosures
No funding was obtained for the authors to conduct this research. The authors have no conflicts of interest that are relevant to the content of this article.
Author contributions
Mona W. Ayad suggested the research project, established the laboratory, participated in writing the article, and is the guarantor for the overall content of this article. Amany A. Elbanna participated in establishing the research project, provided access to patients, performed examinations and ultrasonography, and participated in writing the article. Dalia A. Elneily performing laboratory work and participated in revising the article. Amany S. Sakr performed laboratory work, such as sampling, participated in data collection from patients and control subjects, and participated in writing the article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ayad, M.W., Elbanna, A.A., Elneily, D.A. et al. Association of BAFF −871C/T Promoter Polymorphism with Hepatitis C-Related Mixed Cryoglobulinemia in a Cohort of Egyptian Patients. Mol Diagn Ther 19, 99–106 (2015). https://doi.org/10.1007/s40291-015-0134-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-015-0134-7