Abstract
Introduction
Drug safety remains a top global public health concern. An increase in the number of data sources available has increased the complexity of pharmacovigilance operations, so the US FDA has created draft guidance focusing on optimizing drug safety data for well-characterized medicines. However, to date, no data demonstrating changes in reports have been presented.
Objectives
This study provided data assessing changes in individual case safety reports (ICSRs) and aggregate reports (ARs) for large biopharmaceutical companies from 2007 to 2017. This study also evaluated current trends on the use of advanced machine and deep learning in order to process all data captured for ICSRs as well as opinions from industry thought leaders on creating a sustainable case-processing operation.
Methodology
Using data captured from Navitas Life Science’s annual pvnet® benchmark, we calculated workload indicators characterizing pharmacovigilance operations for large biopharmaceutical organizations. Workload indicators included the number of ICSRs by organization, the number of ARs, and the number and types of data sources used. We also conducted structured in-depth interviews with seven biopharmaceutical executives to discover the reasons for changes in workload indicators across time as well as current strategies for increasing efficiencies in drug safety reporting.
Results
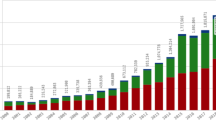
The median number of ICSRs increased from 84,960 cases in 2007 to over 200,000 cases in 2017; this increase was largely attributable to an increase in both nonserious cases and follow-up cases. Member companies reported using 12 ± 3 data sources for case identification. The number of ARs also increased from a median of 70 reports in 2007 to 258 reports in 2017. To address these increases, 61% of the biopharmaceutical organizations we surveyed planned to adopt machine learning for full ICSR processing; however, as of 2018, none of the organizations surveyed had mechanisms in place.
Conclusion
This study demonstrated that pharmacovigilance departments are currently burdened by ever-increasing case volumes. With increased guidance from regulatory agencies, as well as improvements in artificial intelligence and natural language processing, biopharmaceutical organizations must determine the most resource-efficient and sustainable methods to process the growing volume of cases.









Similar content being viewed by others
Change history
18 December 2019
The correct name of the second author should be “Moritz Fehrle”, and not “Mortiz Fehrle” as given in the original publication of the article.
18 December 2019
The correct name of the second author should be ���Moritz Fehrle���, and not ���Mortiz Fehrle��� as given in the original publication of the article.
References
Larizgoitia I, Bouesseau M-C, Kelley E. WHO efforts to promote reporting of adverse events and global learning. J Public Health Res. 2013;2(3):e29.
Pontes H, Clément M, Rollason V. Safety signal detection: the relevance of literature review. Drug Saf. 2014;37(7):471–9.
Pham JC, Gianci S, Battles J, et al. Establishing a global learning community for incident-reporting systems. Qual Saf Health Care. 2010;19(5):446–51.
Bahk CY, Goshgarian M, Donahue K, et al. Increasing patient engagement in pharmacovigilance through online community outreach and mobile reporting applications: an analysis of adverse event reporting for the essure device in the US. Pharm Med. 2015;29(6):331–40.
Beckmann J, Hagemann U, Bahri P, et al. Teaching pharmacovigilance: the WHO-ISoP core elements of a comprehensive modular curriculum. Drug Saf. 2014;37(10):743–59.
Wallington SF, Dash C, Sheppard VB, et al. Enrolling minority and underserved populations in cancer clinical research. Am J Prev Med. 2016;50(1):111–7.
Talbot JC, Nilsson BS. Pharmacovigilance in the pharmaceutical industry. Br J Clin Pharmacol. 1998;45(5):427–31.
Annual Report on EudraVigilance for the European Parliament, the Council and the Commission. European Medicines Agency. 2018.
Dal Pan GJ. Ongoing challenges in pharmacovigilance. Drug Saf Int J Med Toxicol Drug Exp. 2014;37(1):1–8.
CFDA Sees Increase in Adverse Event Reports. FDANews. The QMN Weekly Bulletin Web site. https://www.fdanews.com/articles/181935-cfda-sees-increase-in-adverse-event-reports. Published 2017. Updated May 26, 2017. Accessed 12 Febr 2019.
He W, Yao D, Hu Y, Dai H. Analysis of a pharmacist-led adverse drug event management model for pharmacovigilance in an academic medical center hospital in China. Ther Clin Risk Manag. 2018;14:2139–47.
Mitta I. Current status on Adverse Event Reporting in Japan. In: Paper presented at: 6th Joint Conference of Taiwan and Japan on Medical Products Regulation; October 11, 2018, 2018.
Uppsala Monitoring Center: Annual report July 2017-June 2018. Uppsala, Sweden: Uppsala Monitoring Center; World Health Organization;2018
Sessa M, di Mauro G, Mascolo A, et al. Pillars and pitfalls of the new pharmacovigilance legislation: consequences for the identification of adverse drug reactions deriving from abuse, misuse, overdose, occupational exposure, and medication errors. Front Pharmacol. 2018;9:611.
Toki T, Ono S. Spontaneous reporting on adverse events by consumers in the United States: an analysis of the food and drug administration adverse event reporting system database. Drugs Real World Outcomes. 2018;5(2):117–28.
Pharmacovigilance Market Dynamics and Service Provider Benchmarking. USA: Industry Standard Research (ISR) Reports; November 2014 2014.
Getz KA, Stergiopoulos S, Kaitin KI. Evaluating the completeness and accuracy of MedWatch data. Am J Ther. 2014;21(6):442–6.
Francisca RDC, Zomerdijk IM, Sturkenboom M, Straus S. Measuring the impact of the 2012 European pharmacovigilance legislation on additional risk minimization measures. Expert Opin Drug Saf. 2018;17(10):975–82.
Vermeer NS, Straus SM, Mantel-Teeuwisse AK, et al. Traceability of biopharmaceuticals in spontaneous reporting systems: a cross-sectional study in the FDA Adverse Event Reporting System (FAERS) and EudraVigilance databases. Drug Saf Int J Med Toxicol Drug Exp. 2013;36(8):617–25.
Directive 2012/26/EU of the European Parliament and of the council of 25 October 2012 amending Directive 2001/83/EC as regards pharmacovigilance. Off J Eur Union. 2012:4
Gronning N. Data management in a regulatory context. Front Med (Lausanne). 2017;4:114.
Submitting Documents Using Real-World Data and Real-World Evidence to FDA for Drugs and Biologics: Guidance for Industry. In: Services USDoHaH, Administration FaD, (CDER) CfDEaR, (CBER) CfBEaR, eds. Rockville, MD: Federal Register; 2019:8.
Price J. Pharmacovigilance in crisis: drug safety at a crossroads. Clin Ther. 2018;40(5):790–7.
Legal framework: Pharmacovigilance. European Medicines Agency. https://www.ema.europa.eu/en/human-regulatory/overview/pharmacovigilance/legal-framework-pharmacovigilance. Published 2015. Updated May 18, 2015. Accessed 12 Febr 2019.
Good pharmacovigilance practices. European Medicines Agency. https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/good-pharmacovigilance-practices#introduction-section. Published 2019. Accessed 12 Feb 2019.
Patadia VK, Nimke D, Stefansdottir G, et al. A business intelligence solution to pharmacovigilance signal tracking and management: one mid-size pharma’s experience. Pharm Med. 2015;29(4):197–201.
Reyes SA, King TA, Fei K, Franco R, Bickell NA. Factors affecting the completion of adjuvant chemotherapy in early-stage breast cancer. Ann Surg Oncol. 2016;23(5):1537–42.
Bate A, Reynolds RF, Caubel P. The hope, hype and reality of Big Data for pharmacovigilance. Ther Adv Drug Saf. 2018;9(1):5–11.
Beninger P. Pharmacovigilance: an overview. Clin Ther. 2018;40(12):1991–2004.
Bhangale R, Vaity S, Kulkarni N. A day in the life of a pharmacovigilance case processor. Perspect Clin Res. 2017;8(4):192–5.
Caster O, Dietrich J, Kurzinger ML, et al. Assessment of the utility of social media for broad-ranging statistical signal detection in pharmacovigilance: results from the WEB-RADR project. Drug Saf Int J Med Toxicol Drug Exp. 2018;41(12):1355–69.
Chapman AB, Peterson KS, Alba PR, DuVall SL, Patterson OV. Detecting adverse drug events with rapidly trained classification models. Drug Saf Int J Med Toxicol Drug Exp. 2019;42(1):147–56.
Dandala B, Joopudi V, Devarakonda M. Adverse drug events detection in clinical notes by jointly modeling entities and relations using neural networks. Drug Saf Int J Med Toxicol Drug Exp. 2019;42(1):135–46.
Donzanti BA. Pharmacovigilance is everyone’s concern: let’s work it out together. Clin Ther. 2018;40(12):1967–72.
Furlan G, van Leeuwen B, Edwards B. Considerations for good pharmacovigilance outsourcing practices. Pharm Med. 2017;31(2):75–80.
Jagannatha A, Liu F, Liu W, Yu H. Overview of the first natural language processing challenge for extracting medication, indication, and adverse drug events from electronic health record notes (MADE 1.0). Drug Saf Int J Med Toxicol Drug Exp. 2019;42(1):99–111.
Liu F, Jagannatha A, Yu H. Towards drug safety surveillance and pharmacovigilance: current progress in detecting medication and adverse drug events from electronic health records. Drug Saf Int J Med Toxicol Drug Exp. 2019;42(1):95–7.
Wunnava S, Qin X, Kakar T, Sen C, Rundensteiner EA, Kong X. Adverse drug event detection from electronic health records using hierarchical recurrent neural networks with dual-level embedding. Drug Saf Int J Med Toxicol Drug Exp. 2019;42(1):113–22.
Yang X, Bian J, Gong Y, Hogan WR, Wu Y. MADEx: a system for detecting medications, adverse drug events, and their relations from clinical notes. Drug Saf Int J Med Toxicol Drug Exp. 2019;42(1):123–33.
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. ICH harmonised guideline E19: optimisation of safety data collection. Draft version, 3 April 2019. https://www.fda.gov/media/128313/download. Accessed 30 October 2019.
Abatemarco D, Perera S, Bao SH, et al. Training augmented intelligent capabilities for pharmacovigilance: applying deep-learning approaches to individual case safety report processing. Pharm Med. 2018;32(6):391–401.
Mockute R, Desai S, Perera S, et al. Artificial intelligence within pharmacovigilance: a means to identify cognitive services and the framework for their validation. Pharm Med. 2019;33(2):109–20.
Vaismoradi M, Turunen H, Bondas T. Content analysis and thematic analysis: implications for conducting a qualitative descriptive study. Nurs Health Sci. 2013;15(3):398–405.
Hauben M, Reynolds R, Caubel P. Deconstructing the pharmacovigilance hype cycle. Clin Ther. 2018;40(12):1981–1990.e1983.
Harinstein L, Kalra D, Kortepeter CM, Muñoz MA, Wu E, Pan GJD. Evaluation of postmarketing reports from industry-sponsored programs in drug safety surveillance. Drug Saf. 2018;42:649–55.
Misu T, Kortepeter CM, Muñoz MA, Wu E, Dal Pan GJ. An evaluation of “Drug Ineffective” postmarketing reports in drug safety surveillance. Drugs Real World Outcomes. 2018;5(2):91–9.
Acknowledgements
The authors acknowledge Marie-Claire Wilson and Pete Boyd for their contributions to the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to conduct this study or prepare this manuscript.
Conflict of interest
SS is a paid employee of Foundation Medicine, Inc. but was employed at the Tufts Center for the Study of Drug Development at the time the study was conducted. MF is a paid employee of Bayer AG. PC is a paid employee of Pfizer, Inc. LT and LJ are paid employees of Navitas Life Sciences. No companies contributed to or influenced the data analysis, study conduct, or writing of the manuscript. The manuscript reflects the authors’ personal opinions and contributions.
Ethical Approval
This article does not contain any studies with human or animal subjects performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stergiopoulos, S., Fehrle, M., Caubel, P. et al. Adverse Drug Reaction Case Safety Practices in Large Biopharmaceutical Organizations from 2007 to 2017: An Industry Survey. Pharm Med 33, 499–510 (2019). https://doi.org/10.1007/s40290-019-00307-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40290-019-00307-x