Abstract
Introduction
The approval of orphan anticancer drugs is encouraged in Japan to meet high social demand. However, approval lag and its main component, submission lag, between the USA and Japan continues to be an issue for these drugs.
Objectives
We aimed to identify the main reasons for submission lags with orphan anticancer drugs, to compare these between orphan and non-orphan anticancer drugs, and to identify factors associated with the main reasons for submission lags for orphan anticancer drugs.
Methods
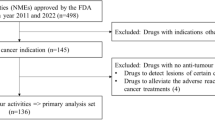
We investigated anticancer drugs approved in Japan between April 2004 and December 2017 using publicly available information. We used Pearson’s product moment correlation coefficient to identify correlations between submission lag and initiation lag or development-time lag, and we used the Mann–Whitney U test to compare contributors to submission lags for both orphan and non-orphan anticancer drugs. We used multiple regression analysis to identify potential factors associated with the main reasons for submission lags for orphan anticancer drugs at the indication level. Independent variables were selected using backward/forward stepwise selection according to the Akaike information criterion.
Results
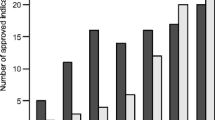
In Japan, the number of approved indications for orphan anticancer drugs consistently increased between 2004–2007 and 2016–2017. The median submission lag for orphan anticancer drugs in 2016–2017 was 515.0 days [interquartile range (IQR) 182.0–999.0], and this lag was significantly correlated with the initiation lag (correlation coefficient 0.77, P < 0.001) but not with the development-time lag (correlation coefficient − 0.031; P = 0.82). The initiation lag was significantly longer for orphan than for non-orphan anticancer drugs [median 1428.0 (IQR 890.8–2655.8) vs. 1178.0 days (369.0–1874.0); P = 0.033]. Cytotoxic drugs were significantly associated with a longer initiation lag (coefficient 2011.8; P = 0.0023), whereas designation as a breakthrough therapy in the USA was significantly associated with a shorter initiation lag (coefficient − 1272.3; P = 0.020).
Conclusions
The initiation lag for orphan anticancer drugs was the main factor affecting submission lag and was longer than that for non-orphan drugs. Of the factors associated with initiation lags, designation as a breakthrough therapy (or the possibility of such a designation) in the USA may lead to earlier initiation of clinical development of an orphan anticancer drug in Japan. In turn, this may reduce the submission lag.





Similar content being viewed by others
References
Tsuji K, Tsutani K. Approval of new drugs 1999–2007: comparison of the US, the EU and Japan situations. J Clin Pharm Ther. 2010;35(3):289–301.
Ministry of Health, Labour and Welfare. Ethnic factors in the acceptability of foreign clinical data. (in Japanese) 1998. https://www.pmda.go.jp/files/000156571.pdf Accessed 15 Aug 2018.
Ministry of Health, Labour and Welfare. Guidance for establishing safety in first-in-human studies during drug development. 2005. https://www.pmda.go.jp/files/000208201.pdf Accessed 15 Aug 2018.
Ministry of Health, Labour and Welfare. Basic principles on global clinical trials. 2007. https://www.pmda.go.jp/files/000157900.pdf Accessed 15 Aug 2018.
Ito Y, Narimatsu H, Fukui T, Fukao A, Yoshioka T. Critical review of ‘Public domain application’: a flexible drug approval system in Japan. Ann Oncol. 2013;24(5):1297–305.
Shimazawa R, Ikeda M. Japanese regulatory system for approval of off-label drug use: evaluation of safety and effectiveness in literature-based applications. Clin Ther. 2012;34(10):2104–16.
Ito T, Urushihara H, Matsushima Y, Nakajima K, Kurokawa T. Mode of regulatory applications of drugs used off-label reviewed by the evaluation committee on unapproved or off-labeled drugs with high medical needs. Jpn J Clin Pharmacol Ther. 2015;46(5):233–41 (in Japanese).
Ministry of Health, Labour and Welfare. Strategy of SAKIGAKE. 2014. https://www.mhlw.go.jp/english/policy/health-medical/pharmaceuticals/dl/140729-01-01.pdf Accessed 15 Aug 2018.
Ministry of Health, Labour and Welfare. Implementation of a conditional early approval system for pharmaceutical products. 2017. https://www.pmda.go.jp/files/000222439.pdf Accessed 15 Aug 2018.
Yonemori K, Hirakawa A, Ando M, Hirata T, Yunokawa M, Shimizu C, et al. The notorious “drug lag” for oncology drugs in Japan. Invest New Drugs. 2011;29(4):706–12.
Blay JY, Coindre JM, Ducimetiere F, Ray-Coquard I. The value of research collaborations and consortia in rare cancers. Lancet Oncol. 2016;17(2):e62–9.
Gatta G, van der Zwan JM, Casali PG, Siesling S, Dei Tos AP, Kunkler I, et al. Rare cancers are not so rare: the rare cancer burden in Europe. Eur J Cancer. 2011;47(17):2493–511.
Heemstra HE, van Weely S, Buller HA, Leufkens HG, de Vrueh RL. Translation of rare disease research into orphan drug development: disease matters. Drug Discov Today. 2009;14(23–24):1166–73.
Ministry of Health, Labour and Welfare. Overview of orphan drug/medical device designation system. (in Japanese) 2015. http://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000068484.html Accessed 15 Aug 2018.
Boyd N, Dancey JE, Gilks CB, Huntsman DG. Rare cancers: a sea of opportunity. Lancet Oncol. 2016;17(2):e52–61.
Kumar Kakkar A, Dahiya N. The evolving drug development landscape: from blockbusters to niche busters in the orphan drug space. Drug Dev Res. 2014;75(4):231–4.
Gaddipati H, Liu K, Pariser A, Pazdur R. Rare cancer trial design: lessons from FDA approvals. Clin Cancer Res. 2012;18(19):5172–8.
Nakayama H, Tsukamoto K. Unique characteristics of regulatory approval and pivotal studies of orphan anticancer drugs in Japan. Invest New Drugs. 2018;36(4):702–8.
Maeda H, Kurokawa T. Recent trends for drug lag in clinical development of oncology drugs in Japan: does the oncology drug lag still exist in Japan? Int J Clin Oncol. 2015;20(6):1072–80.
Kawabata-Shoda E, Masuda S, Kimura H. Anticancer drug development from traditional cytotoxic to targeted therapies: evidence of shorter drug research and development time, and shorter drug lag in Japan. J Clin Pharm Ther. 2012;37(5):547–52.
Nakayama H, Matsumaru N, Tsukamoto K. The drug lag and associated factors for orphan anticancer drugs in Japan compared to the United States. Invest New Drugs. 2018. https://doi.org/10.1007/s10637-018-0612-y.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013;48(3):452–8.
Hirai Y, Kinoshita H, Kusama M, Yasuda K, Sugiyama Y, Ono S. Delays in new drug applications in Japan and industrial R&D strategies. Clin Pharmacol Ther. 2010;87(2):212–8.
Ueno T, Asahina Y, Tanaka A, Yamada H, Nakamura M, Uyama Y. Significant differences in drug lag in clinical development among various strategies used for regulatory submissions in Japan. Clin Pharmacol Ther. 2014;95(5):533–41.
Maeda H, Kurokawa T. Differences in maximum tolerated doses and approval doses of molecularly targeted oncology drug between Japan and Western countries. Invest New Drugs. 2014;32(4):661–9.
US Food and Drug Administration. Guidance for industry expedited programs for serious conditions—drugs and biologics. 2014. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM358301.pdf Accessed 15 Aug 2018.
Tanaka M, Matsumaru N, Tsukamoto K. Influence of breakthrough therapy designation in the United States on oncology drug development timelines in Japan. Pharm Med. 2018;32(3):201–7.
Pharmaceuticals and Medical Devices Agency. Review report for clofarabine. (in Japanese) 2013. http://www.pmda.go.jp/drugs/2013/P201300047/34053100_22500AMX00882000_A100_3.pdf Accessed 15 Aug 2018.
US Food and Drug Administration. Medical Review(s) for clofarabine. 2004. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/21-673_Clolar_medr.PDF. Accessed 15 Aug 2018.
Sanofi (Genzyme, a Sanofi Company). A study of clofarabine in Japanese patients with acute myeloid leukemia (AML). ClinicalTrials.gov. 2014.https://www.clinicaltrials.gov/ct2/show/NCT01090167Accessed 15 Aug 2018.
Acknowledgements
The authors express their gratitude to Makoto Tanaka for his useful suggestions and to Katsuya Nakano for his review of the study from a regulatory affairs viewpoint.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to conduct this study or prepare this manuscript.
Conflict of Interest
Hiroki Nakayama is an employee of Astellas Pharma Inc. Naoki Matsumaru and Katsura Tsukamoto have no conflicts of interest that are directly relevant to the content of this article.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nakayama, H., Matsumaru, N. & Tsukamoto, K. Delays in New Drug Applications and Associated Factors for Orphan Anticancer Drugs in Japan Compared with the USA. Pharm Med 32, 403–412 (2018). https://doi.org/10.1007/s40290-018-0257-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40290-018-0257-3