Abstract
Objective
This study reviews the use of different study designs used to generate evidence to justify drug withdrawals for safety reasons in the United States (US). Secondarily it examines the most common reasons for withdrawing drugs, how long the withdrawn drugs were on the market and uses statistical modeling to estimate the risk of withdrawal over time.
Methods
A list of drugs withdrawn from the US market for safety reasons was generated, along with the corresponding period of time each drug was marketed. Evidence used to justify the withdrawal was obtained from searching Drugs@FDA and PubMed, and evidence was classified according to the study design used to generate the evidence. The number of drugs withdrawn was plotted as a function of how long they were marketed and a mathematical model was derived from this set of data to calculate the mean time the drugs were marketed before withdrawal.
Results
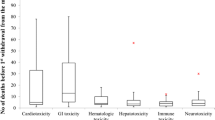
A total of 34 drugs were withdrawn from the market for safety reasons in the US from 1976 to 2010. Nineteen of the 34 withdrawals relied only on case reports. Randomized and non-randomized studies were identified in 15 withdrawals and were increasingly used over the period of time examined. The median length of time that drugs were on the market was 3.4 years [interquartile range (IQR), 1.2, 10.4 years] with a mode at 1 year. The longest marketing period before withdrawal in the current data set was 53 years. Most drugs were withdrawn for either arrhythmias, hepato-renal failure or other cardiovascular problems.
Conclusions
The evidence used to justify safety withdrawals is dominated by case reports although there is an increased use of comparative studies.


Similar content being viewed by others
References
Guyatt G, Oxman A, Sultan S, Glasziou P, Akl E, Alonso-Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011;64:1311–6.
Howick J, Chalmers I, Glasziou P, Greenhalgh T, Heneghan C, Liberati A, et al. Explanation of the 2011 Oxford Centre for Evidence-Based Medicine (OCEBM) Levels of Evidence (Background Document). Oxford Centre for Evidence-Based Medicine; 2011. http://www.cebm.net/index.aspx?o=5653. Accessed 16 Aug 2016.
Darrow J, Kesselheim A. Drug development and FDA approval. N Engl J Med. 2014;370:e39.
Junod S. FDA and clinical drug trials: a short history: US Food and Drug Administration; 2016. http://www.fda.gov/AboutFDA/WhatWeDo/History/Overviews/ucm304485.htm. Accessed 25 Aug 2016.
Graham D, Campen D, Hui R, Spence M, Cheetham C, Levy G, et al. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study. Lancet. 2005;365:475–81.
Onakpoya I, Heneghan C, Aronson J. Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: a systematic review of the world literature. BMC Med. 2016;14:10.
Singh S, Loke Y. Drug safety assessment in clinical trials: methodological challenges and opportunities. Trials. 2012;13:138.
Ray W. Population-based studies of adverse drug effects. N Engl J Med. 2003;349:1592–4.
Lexchin J. Postmarket safety in Canada: are significant therapeutic advances and biologics less safe than other drugs? A cohort study. BMJ Open. 2014;4:e004289.
Vardi M, Perez J, Griffin P, Burke D, Yeh R, Cutlip D. Usefulness of postmarket studies to evaluate long-term safety of coronary eluting stents (from the ENDEAVOR and PROTECT Programs). Am J Cardiol. 2014;114:528–33.
Martin K, Begaud B, Latry P, Miremont-Salame G, Fourrier A, Moore N. Differences between clinical trials and postmarketing use. Br J Clin Pharmacol. 2004;57:86–92.
LaRochelle P. Basic learning concepts in EBM: the bidimentional hierarchy of evidence. Evid Based Med. 2014;19:83–4.
Frank C, Himmelstein D, Woolhandler S, Bor D, Wolfe S, Heymann O, et al. Era of faster FDA drug approval has also seen increased black-box warnings and market withdrawals. Health Aff. 2014;33:1453–9.
US FDA. Index to drug-specific information. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm111085.htm. Accessed 25 Aug 2016.
US FDA. Recalls, market withdrawal, and safety alerts. http://www.fda.gov/Safety/recalls/default.htm. Accessed 25 Aug 2016.
US FDA. Drugs@FDA. http://www.accessdata.fda.gov/Scripts/cder/Drugsatfda/. Accessed 25 Aug 2016
US Food and Drug Administration. CDER 2005 report to the nation: improving public health through human drugs. Rockville: FDA; 2005.
Qureshi Z, Seoane-Vazquez E, Rodriguez-Monguio R, Stevenson K, Szeinbach S. Market withdrawal of new molecular entities approved in the United States from 1980 to 2009. Pharmacoepidemiol Drug Saf. 2011;20:772–7.
Wysowski D, Swartz L. Adverse drug event surveillance and drug withdrawals in the United States, 1969–2002: the importance of reporting suspected reactions. Arch Intern Med. 2005;165:1363–9.
Lasser K, AlLen P, Woolhandler S, Himmelstein D, Wolfe S, Bor D. Timing of new black box warnings and withdrawals for prescription medications. JAMA. 2002;287:2215–20.
Issa A, Phillips K, Van Bebber S, Nidamarthy H, Lasser K, Haas J, et al. Drug withdrawals in the United States: a systematic review of the evidence and analysis of trends. Curr Drug Saf. 2007;2:177–85.
Obias-Manno D, Scott P, Kaczmarczyk J, Miller M, Pinnow E, Lee-Bishop L, et al. The Food and Drug Administration Office of Women’s Health: impact of science on regulatory policy. J Womens Health. 2007;16:807–17.
Huang S, Lesko L. Drug-drug, drug-dietary supplement, and drug-citrus fruit and other food interactions: what have we learned? J Clin Pharmacol. 2004;44:559–69.
Abraham J, Davis C. A comparative analysis of drug safety withdrawals in the UK and the US (1971–1992): implications for current regulatory thinking and policy. Soc Sci Med. 2005;61:881–92.
Nardi A, Schemper M. Comparing Cox and parametric models in clinical studies. Stat Med. 2003;22(23):3597–610.
US Food and Drug Administration. CDER 2003 report to the nation: improving public health through human drugs. Rockville: FDA; 2003.
US Food and Drug Administration. FDA announces discontinued marketing of GI drug Zelnorm, for safety reasons. Rockville: FDA; 2007. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm108879.htm. Accessed 25 Aug 2016.
Arnaiz J, Carne X, Riba N, Codina C, Ribas J, Trilla A. The use of evidence in pharmacovigilance. Case reports as the reference source for drug withdrawals. Eur J Clin Pharmacol. 2001;57:89–91.
Clarke A, Deeks J, Shakir S. An assessment of the publicly disseminated evidence of safety used in decisions to withdraw medicinal products from the UK and US markets. Drug Saf. 2006;29:175–81.
McNaughton R, Huet G, Shakir S. An investigation into drug products withdrawn from the EU market between 2002 and 2011 for safety reasons and the evidence used to support the decision-making. BMJ Open. 2014;4:e004221.
Olivier P, Montastruc J-L. The nature of the scientific evidence leading to drug withdrawals for pharmacovigilance reasons in France. Pharmacoepidemiol Drug Saf. 2006;15:808–12.
Paludetto M-N, Olivier-Abbal P, Montastruc J-L. Is spontaneous reporting always the most important information supporting drug withdrawals for pharmacovigilance reasons in France? Pharmacoepidemiol Drug Saf. 2012;21:1289–94.
Onakpoya I, Heneghan C, Aronson J. Delays in the post-marketing withdrawal of drugs to which deaths have been attributed: a systematic investigation and analysis. BMC Med. 2015;13:26.
Huang S-M. The role of drug transporters in drug safety—perspsectives from the FDA New Orleans: American Association of Pharmaceutical Scientists; 2010 Nov 18. http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/UCM237145.pdf. Accessed 25 Aug 2016.
Scholl J, van de Ven P, van Puijenbroek E. Parametric time-to-onset models were developed to improve causality assessment of adverse drug reactions from antidiabetic drugs. J Clin Epidemiol. 2015;68:1423–31.
Cornelius V, Sauzet O, Evans S. A signal detection method to detect adverse drug reactions using a parametric time-to-event model in simulated cohort data. Drug Saf. 2012;35:599–610.
Sauzet O, Carvajal A, Escudero A, Molokhia M, Cornelius V. Illustration of the weibull shape parameter signal detection tool using electronic healthcare record data. Drug Saf. 2013;36:995–1006.
Van Holle L, Zeinoun Z, Bauchau V, Verstraeten T. Using time-to-onset for detecting safety signals in spontaneous reports of adverse events following immunization: a proof of concept study. Pharmacoepidemiol Drug Saf. 2012;21:603–10.
Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med. 2010;7:e1000326.
The Canadian Cooperative Study Group. A randomized trial of aspirin and sulfinpyrazone in threatened stroke. N Engl J Med. 1978;299:53–9.
The SPS3 Investigators. Effects of clopidrogel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817–25.
Khoury M, Ioannidis J. Big data meets public health. Science. 2014;346:1054–5.
FDA. Phenylpropanolamine withdrawal. 2000. http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm150738.htm. Accessed 25 Aug 2016.
FDA. Propoxyphen withdrawal. 2010 [updated 11 Apr 2011]. http://www.fda.gov/Drugs/DrugSafety/ucm234338.htm. Accessed 25 Aug 2016.
Brenot F, Herve P, Petitpretz P, Parent F, Duroux P, Simonneau G. Primary pulmonary hypertension and fenfluramine use. Br Heart J. 1993;70(6):537–41.
Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, et al. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337(9):581–8.
Abenhaim L, Moride Y, Brenot F, Rich S, Benichou J, Kurz X, et al. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. International Primary Pulmonary Hypertension Study Group. N Engl J Med. 1996;335(9):609–16.
FDA. Azaribine withdrawal. 1977. http://www.fda.gov/ohrms/dockets/ac/98/briefingbook/1998-3454B1_03_WL03.pdf. Accessed 25 Aug 2016.
de Abajo FJ, Rodriguez LA. Risk of ventricular arrhythmias associated with nonsedating antihistamine drugs. Br J Clin Pharmacol. 1999;47(3):307–13.
FDA. Pemoline withdrawal. 2005. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm126461.htm. Accessed 25 Aug 2016.
FDA. Ticrynafen withdrawal. 1980. http://www.fda.gov/ohrms/dockets/ac/98/briefingbook/1998-3454B1_03_WL53.pdf. Accessed 25 Aug 2016.
FDA. Zomepirac withdrawal. 1983. http://www.fda.gov/ohrms/dockets/ac/98/briefingbook/1998-3454B1_03_WL59.pdf. Accessed 25 Aug 2016.
FDA. Nomifensine withdrawal. 1986. Available from: http://www.fda.gov/ohrms/dockets/ac/98/briefingbook/1998-3454B1_03_WL35.pdf. Accessed 25 Aug 2016.
FDA. Benoxaprofen withdrawal. 1982. http://www.fda.gov/ohrms/dockets/ac/98/briefingbook/1998-3454B1_03_WL04.pdf. Accessed 25 Aug 2016.
FDA. Suprofen warning. 1986. http://www.fda.gov/ohrms/dockets/ac/98/briefingbook/1998-3454B1_03_WL47.pdf. Accessed 25 Aug 2016.
FDA. Etretinate withdrawal. 1999. http://www.fda.gov/OHRMS/DOCKETS/98fr/091003e.htm. Accessed 25 Aug 2016.
Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. N Engl J Med. 1989;321(6):406–12.
Schade R, Andersohn F, Suissa S, Haverkamp W, Garbe E. Dopamine agonists and the risk of cardiac-valve regurgitation. N Engl J Med. 2007;356(1):29–38.
Zanettini R, Antonini A, Gatto G, Gentile R, Tesei S, Pezzoli G. Valvular heart disease and the use of dopamine agonists for Parkinson’s disease. N Engl J Med. 2007;356(1):39–46.
FDA. Temafloxacin withdrawal. 1992. http://www.fda.gov/ohrms/dockets/ac/98/briefingbook/1998-3454B1_03_WL49.pdf. Accessed 25 Aug 2016.
FDA. Flosequinan withdrawal. 1993. http://www.fda.gov/ohrms/dockets/ac/98/briefingbook/1998-3454B1_03_WL23.pdf. Accessed 25 Aug 2016.
FDA. Levomethadyl withdrawal. 2003. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm153332.htm. Accessed 25 Aug 2016.
Wysowski DK, Bacsanyi J. Cisapride and fatal arrhythmia. N Engl J Med. 1996;335(4):290–1.
Hill SL, Evangelista JK, Pizzi AM, Mobassaleh M, Fulton DR, Berul CI. Proarrhythmia associated with cisapride in children. Pediatrics. 1998;101(6):1053–6.
Watkins PB, Whitcomb RW. Hepatic dysfunction associated with troglitazone. N Engl J Med. 1998;338(13):916–7.
FDA. Mibefradil withdrawal. 1998. http://www.fda.gov/ohrms/dockets/ac/98/briefingbook/1998-3454B1_03_WL32.pdf. Accessed 25 Aug 2016.
FDA. Cerivastatin withdrawal. 2001. http://www.fda.gov/OHRMS/DOCKETS/98fr/091003e.htm. Accessed 25 Aug 2016.
FDA. Bromfenac withdrawal. 1998. http://www.fda.gov/ohrms/dockets/ac/98/briefingbook/1998-3454B1_03_WL06.pdf. Accessed 25 Aug 2016.
FDA. Grepafloxacin withdrawal. 1999. http://www.fda.gov/ohrms/dockets/ac/00/backgrd/3634b1a_tab5b.htm. Accessed 25 Aug 2016.
James WP, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363(10):905–17.
Padwal R, Li SK, Lau DC. Long-term pharmacotherapy for obesity and overweight. Cochrane Database Syst Rev. 2003(4):CD004094.
Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352(11):1092–102.
Sparr HJ, Mellinghoff H, Blobner M, Noldge-Schomburg G. Comparison of intubating conditions after rapacuronium (Org 9487) and succinylcholine following rapid sequence induction in adult patients. Br J Anaesth. 1999;82(4):537–41.
Kron SS. Severe bronchospasm and desaturation in a child associated with rapacuronium. Anesthesiology. 2001;94(5):923–4.
Camilleri M, Northcutt AR, Kong S, Dukes GE, McSorley D, Mangel AW. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet. 2000;355(9209):1035–40.
FDA. Alosetron withdrawal. 2000. http://www.vidyya.com/archives/1130_2.htm. Accessed 25 Aug 2016.
FDA. Gemtuzumab ozogamicin withdrawal. 2010 [updated 11 Apr 2011]. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2010/ucm216448.htm. Accessed 25 Aug 2016.
FDA. Valdecoxib withdrawal. 2005 [updated 11 Apr 2011]. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124649.htm. Accessed 25 Aug 2016.
FDA. Tegaserod withdrawal. 2007 [updated 11 Apr 2011]. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm108879.htm. Accessed 25 Aug 2016.
FDA. Efalizumab withdrawal. 2009 [updated 11 Apr 2011]. http://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm143347.htm. Accessed 25 Aug 2016.
FDA. Technecium fanolesomab withdrawal. 2005. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm129299.htm. Accessed 25 Aug 2016.
FDA. Natalizumab withdrawal. 2005. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm051761.htm. Accessed 25 Aug 2016.
Birkenfeld AL, Schroeder C, Boschmann M, Tank J, Franke G, Luft FC, et al. Paradoxical effect of sibutramine on autonomic cardiovascular regulation. Circulation. 2002;106(19):2459–65.
Padwal R, Li SK, Lau DC. Long-term pharmacotherapy for obesity and overweight. Cochrane Database Syst Rev. 2004(3):CD004094.
Corvol JC, Anzouan-Kacou JB, Fauveau E, Bonnet AM, Lebrun-Vignes B, Girault C, et al. Heart valve regurgitation, pergolide use, and parkinson disease: an observational study and meta-analysis. Arch Neurol. 2007;64(12):1721–6.
Juni P, Nartey L, Reichenbach S, Sterchi R, Dieppe PA, Egger M. Risk of cardiovascular events and rofecoxib: cumulative meta-analysis. Lancet. 2004;364(9450):2021–9.
Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med. 2000;343(21):1520–8 (2 p following 8).
Meakin GH, Pronske EH, Lerman J, Orr R, Joffe D, Savaree AM, et al. Bronchospasm after rapacuronium in infants and children. Anesthesiology. 2001;94(5):926–7.
Khoshoo V, Edell D, Clarke R. Effect of cisapride on the QT interval in infants with gastroesophageal reflux. Pediatrics. 2000;105(2):E24.
Neuschwander-Tetri BA, Isley WL, Oki JC, Ramrakhiani S, Quiason SG, Phillips NJ, et al. Troglitazone-induced hepatic failure leading to liver transplantation. A case report. Ann Intern Med. 1998;129(1):38–41.
Gitlin N, Julie NL, Spurr CL, Lim KN, Juarbe HM. Two cases of severe clinical and histologic hepatotoxicity associated with troglitazone. Ann Intern Med. 1998;129(1):36–8.
Schwartz S, Raskin P, Fonseca V, Graveline JF. Effect of troglitazone in insulin-treated patients with type II diabetes mellitus. Troglitazone and Exogenous Insulin Study Group. N Engl J Med. 1998;338(13):861–6.
Jones RH, Holtmann G, Rodrigo L, Ehsanullah RS, Crompton PM, Jacques LA, et al. Alosetron relieves pain and improves bowel function compared with mebeverine in female nonconstipated irritable bowel syndrome patients. Aliment Pharmacol Ther. 1999;13(11):1419–27.
Kamali F, Edwards C. Possible role of metabolite in flosequinan-related mortality. Clin Pharmacokinet. 1995;29(6):396–403.
Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324(12):781–8.
Arnaiz JA, Carne X, Riba N, Codina C, Ribas J, Trilla A. The use of evidence in pharmacovigilance. Case reports as the reference source for drug withdrawals. Eur J Clin Pharmacol. 2001;57(1):89–91.
Clarke A, Deeks JJ, Shakir SA. An assessment of the publicly disseminated evidence of safety used in decisions to withdraw medicinal products from the UK and US markets. Drug Saf. 2006;29(2):175–81.
Olivier P, Montastruc JL. The nature of the scientific evidence leading to drug withdrawals for pharmacovigilance reasons in France. Pharmacoepidemiol Drug Saf. 2006;15(11):808–12.
McNaughton R, Huet G, Shakir S. An investigation into drug products withdrawn from the EU market between 2002 and 2011 for safety reasons and the evidence used to support the decision-making. BMJ Open 2014;4:e004221. doi:10.1136/bmjopen-2013-004221.
Paludetto M-N, Olivier-Abbal P, Montastruc J-L. Is spontaneous reporting always the most important information supporting drug withdrawals for pharmacovigilance reasons in France? Pharmacoepidemiol Drug Saf. 2012;21(12):1289–94.
Acknowledgments
The initial guidance for this project was provided by Professor Paul Glasziou, who made recommendations about defining and limiting the research focus. He also provided advice on how to select outcomes and present the results. Dr. Amanda Burls supervised Dr. Pierre La Rochelle throughout the entire research project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this study.
Conflicts of interest
In 2015 and 2016 Joel Lexchin received payment for (a) participating in a panel discussion on the introduction of drug insurance in Canada; (b) being a consultant to a project looking at indication-based prescribing; and (c) being a consultant to a project that evaluated which drugs should be provided free of charge in a family practice setting. Pierre La Rochelle and David Simonyan have no conflicts of interest to declare.
Ethical approval
No ethical approval was necessary as this study was based on publicly available data.
Rights and permissions
About this article
Cite this article
La Rochelle, P., Lexchin, J. & Simonyan, D. Analysis of the Drugs Withdrawn from the US Market from 1976 to 2010 for Safety Reasons. Pharm Med 30, 277–289 (2016). https://doi.org/10.1007/s40290-016-0159-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40290-016-0159-1