Abstract
Objective
The aim of this study was to assess whether S-1 might represent a valuable therapeutic option for patients with advanced gastric cancer in comparison to other available therapies (5-FU, capecitabine), considering both costs and outcomes associated with the different therapeutic strategies. The perspective of the analysis was that of the Italian National Health Service (NHS).
Design
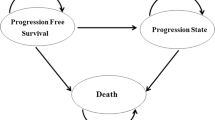
The economic analysis was carried out using different approaches, taking into account the available clinical evidence. A cost-minimization analysis was based on the non-inferiority hypothesis of S-1 and capecitabine versus 5-FU (in terms of overall survival) observed in the FLAGS study (2010) and in Kang et al. (2010). Moreover, a Markov model was developed to evaluate costs of S-1 versus 5-FU per treatment failure avoided. The model was based on three health states (advanced gastric cancer, progression after treatment failure, death) and combined efficacy data from a published clinical trial (FLAGS) with costs of therapies and other direct costs. Costs and effects were both discounted at 3.5%. Deterministic and probabilistic sensitivity analyses were carried out to test the robustness of the results. A further analysis was conducted in order to assess the potential budget impact of S-1 on the Italian NHS.
Results
Cost-minimization analysis showed the possibility for the NHS to obtain greater cost-savings by treating patients with S-1 rather than with capecitabine. The average total cost per patient amounted to €6,689 for S-1, €7,464 for capecitabine and €18,207 for 5-FU. The cost-effectiveness analysis revealed that S-1 is dominant, if compared to 5-FU, because of cost-savings of about €6,243 for approximately 0.11 cases of treatment failure avoided over a lifetime horizon. These savings were mainly due to the different administration setting (S-1 oral therapy, 5-FU infusion), and to the better safety profile of S-1 compared to 5-FU.
Conclusions
The introduction of S-1 in the treatment of advanced gastric cancer would result in savings for the Italian NHS, mainly due to the reduction of adverse events and hospitalizations. Further studies are warranted in order to carry out a direct comparison with capecitabine, which represents the most appropriate comparator for the cost-effectiveness analysis.


Similar content being viewed by others
Notes
Teysuno® (S-1) ha ricevuto l’Autorizzazione all’Immissione in Commercio da parte dell’EMA il 14 marzo 2011 ed è indicato negli adulti per il trattamento del carcinoma gastrico avanzato quando somministrato in combinazione con cisplatino.
Bibliografia
Istituto Superiore di Sanità (ISS). Basi Scientifiche per Linee Guida in ambito clinico per le diverse patologie oncologiche. Carcinoma gastrico. 2011. Disponibile online: http://www.iss.it/lgac/docu/index.php?lang=1&tipo=32 (ultimo accesso 3 luglio 2012).
Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917.
World Health Organization (WHO). Cancer. Fact Sheet N∘297. 2011. Disponibile online: http://www.who.int/mediacentre/factsheets/fs297/en/) (ultimo accesso 3 luglio 2012).
Arfè A, Malvezzi M, Bertuccio P, et al. Cancer mortality trend analysis in Italy, 1970–2007. Eur J Cancer Prev. 2011;20:364–74.
Tavolo di lavoro AIOM-AIRTUM. I numeri del cancro in Italia. 2011. Intermedia Editore: Brescia; 2012. Disponibile online: http://www.registri-tumori.it/PDF/AIOM2011/I_numeri_del_cancro_2011.pdf (ultimo accesso 3 luglio 2012).
National Institute for Health and Clinical Excellence (NICE). Draft scope for the proposed appraisal of S-1 for the treatment of advanced gastric cancer. Issue Date: August 2010. Disponibile online: http://www.nice.org.uk/media/7B7/94/AppendixBDraftScopeGastricCancer.pdf (ultimo accesso 3 luglio 2012).
Kang YK, Kang WK, Shin DB, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666–73.
Mackenzie M, Spithoff K, Jonker D, Gastrointestinal Cancer Disease Site Group. Systemic therapy for advanced gastric cancer. Toronto (ON): Cancer Care Ontario (CCO); 2010 Dec 16. 53 p. (Evidence-based series; no. 2-26). Disponibile online: http://www.guideline.gov/content.aspx?id=24140 (ultimo accesso 3 luglio 2012).
Associazione Italiana di Oncologia Medica (AIOM). Linee guida. Neoplasie dello stomaco. Disponibile online: http://www.aiom.it/AttivitE0+Scientifica/Linee+guida/Archivio+2010/Neoplasie%20dello%20stomaco/1,4774,1 (ultimo accesso 3 luglio 2012).
European Medicines Agency (EMA). European Public Assessment Report for tegafur/gimeracil/oteracil (Teysuno®). 2011. EMEAH/C/001242. Disponibile online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/001242/WC500104417.pdf (ultimo accesso 1 marzo 2013).
Ajani JA, Rodriguez W, Bodoky G, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol. 2010;28:1547–53.
Teysuno®—Summary of Product Characteristics (SmPC), November 27, 2012. Disponibile online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR__Product_Information/human/001242/WC500104415.pdf (ultimo accesso 14 giugno 2013).
Giuliani G, Lucioni C, Mazzi S, et al. Valutazione di convenienza economica comparata tra un farmaco orale (capecitabina) e una chemioterapia parenterale a base di 5-FU (regime Mayo) nel trattamento del carcinoma del colon retto metastatizzato. PharmacoEcon, Ital Res Artic. 2002;4:31–8.
Lopatriello S, Amoroso D, Donati S, et al. The CAP-CR study: direct medical costs in Italian metastatic colorectal cancer patients on first-line infusional 5-fluorouracil or oral capecitabine. Eur J Cancer. 2008;44:2615–22.
National Institute for Health and Clinical Excellence (NICE). Guide to the methods of technology appraisal. June, 2008. Disponibile online: http://www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf (ultimo accesso 6 maggio 2013).
Drummond M, Sculpher MJ, Torrance JW, et al. Methods for the economic evaluation of health care programs. Oxford: Oxford University Press; 2005.
Scottish Medicines Consortium (SMC). Tegafur/gimeracil/oteracil 15 mg/4.35 mg/11.8 mg and 20 mg/5.8 mg/15.8 mg hard capsules (Teysuno®). SMC No. (802/12). Disponibile online: http://www.scottishmedicines.org.uk/files/advice/tegafur_gimeracil_oteracil_Teysuno_FINAL_August_2012_amended_11.09.12_for_website.pdf (ultimo accesso 1 marzo 2013).
Specchia ML, Bamfi F, Aguzzi G, et al. Analisi delle conseguenze organizzative dell’introduzione di lapatinib, trattamento orale, nello specifico contesto di cura italiano. Ital J Public Health. 2009;7(1 Suppl 1):60–7.
Ringraziamenti
Questa ricerca è stata supportata da un grant di Nordic Pharma Group. Gli autori dichiarano di non avere conflitti di interesse relativi al contenuto del presente articolo.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruggeri, M., Coretti, S., Carletto, A. et al. Valutazione economica e analisi di budget impact di S-1 (tegafur/gimeracil/oteracil) in pazienti con carcinoma gastrico avanzato. PharmacoEcon Ital Res Artic 15, 65–74 (2013). https://doi.org/10.1007/s40276-013-0007-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40276-013-0007-1