Abstract
Clinical and cost-effectiveness evidence on fulvestrant for untreated hormone-receptor positive locally advanced or metastatic breast cancer was submitted to the single technology appraisal process of the National Institute for Health and Care Excellence by the manufacturer of fulvestrant. The Southampton Health Technology Assessments Centre was commissioned by the National Institute for Health and Care Excellence as an independent Evidence Review Group to critique the company’s submitted evidence. Fulvestrant was compared directly with anastrozole in two randomised controlled trials and was compared indirectly by means of a network meta-analysis with anastrozole, letrozole and tamoxifen. This article summarises the Evidence Review Group’s review of the company’s submission and summarises the guidance the National Institute for Health and Care Excellence Appraisal Committee issued in January 2018. The Evidence Review Group had several concerns, the most important of which related to the degree to which fulvestrant might confer a benefit in overall survival. This was because mature data were not available from the key phase III trial FALCON. The economic model was sensitive to changes in overall survival and the Evidence Review Group considered the incremental cost-effectiveness ratio was uncertain and likely to increase once mature results from FALCON become available. The National Institute for Health and Care Excellence Appraisal Committee concluded that fulvestrant could not be recommended for treating locally advanced or metastatic estrogen-receptor-positive breast cancer in postmenopausal women who have not received previous endocrine therapy.


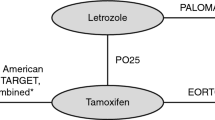
(adapted by the Evidence Review Group)
Similar content being viewed by others
References
Office for National Statistics. Statistical bulletin: cancer registration statistics, England: 2016. 2017. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/datasets/cancerregistrationstatisticscancerregistrationstatisticsengland. Accessed 1 Oct 2018.
Setiawan VW, Monroe KR, Wilkens LR, Kolonel LN, Pike MC, Henderson BE. Breast cancer risk factors defined by estrogen and progesterone receptor status: the multiethnic cohort study. Am J Epidemiol. 2009;169(10):1251–9.
Saha Roy S, Vadlamudi RK. Role of estrogen receptor signaling in breast cancer metastasis. Int J Breast Cancer. 2012;2012:6546988. https://doi.org/10.1155/2012/654698.
Metastatic Breast Cancer Network. Metastatic breast cancer incidence. 2017. http://www.mbcn.org/incidence-and-incidence-rates/. Accessed 1 Oct 2018.
National Collaborating Centre for Cancer. Advanced breast cancer: diagnosis and treatment [CG81]. 2009. https://www.nice.org.uk/guidance/cg81. Accessed 1 Oct 2018.
European Medicines Agency. Summary of product characteristics. Faslodex 2009. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000540/human_med_000786.jsp&mid=WC0b01ac058001d124. Accessed 1 Oct 2018.
National Institute for Health and Care Excellence. Fulvestrant for the treatment of locally advanced or metastatic cancer [TA239]. 2011. https://www.nice.org.uk/guidance/ta239. Accessed 1 Oct 2018.
National Institute for Health Care and Excellence. Single technology appraisal: fulvestrant for untreated hormone-receptor positive locally advanced or metastatic breast cancer. Final scope. Appendix B. 2017. https://www.niceorguk/guidance/gid-ta10106/documents/final-scope. Accessed 1 Oct 2018.
Robertson JF, Llombart-Cussac A, Rolski J, Feltl D, Dewar J, Macpherson E, et al. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: results from the FIRST study. J Clin Oncol. 2009;27(27):4530–5. https://doi.org/10.1200/jco.2008.21.1136.
Robertson JF, Lindemann JP, Llombart-Cussac A, Rolski J, Feltl D, Dewar J, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: follow-up analysis from the randomized ‘FIRST’ study. Breast Cancer Res Treat. 2012;136(2):503–11. https://doi.org/10.1007/s10549-012-2192-4.
Ellis MJ, Llombart-Cussac A, Feltl D, Dewar JA, Jasiowka M, Hewson N, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: overall survival analysis from the phase II FIRST study. J Clin Oncol. 2015;33(32):3781–7. https://doi.org/10.1200/jco.2015.61.5831.
Robertson JF, Bondarenko IM, Trishkina E, Dvorkin M, Panasci L, Manikhas A, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet. 2016;388(10063):2997–3005. https://doi.org/10.1016/s0140-6736(16)32389-3.
Nabholtz JM, Buzdar A, Pollak M, Harwin W, Burton G, Mangalik A, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group. J Clin Oncol. 2000;18(22):3758–67. https://doi.org/10.1200/jco.2000.18.22.3758.
Bonneterre J, Thurlimann B, Robertson JF, Krzakowski M, Mauriac L, Koralewski P, et al. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability Study. J Clin Oncol. 2000;18(22):3748–57. https://doi.org/10.1200/jco.2000.18.22.3748.
Milla-Santos A, Milla L, Portella J, Rallo L, Pons M, Rodes E, et al. Anastrozole versus tamoxifen as first-line therapy in postmenopausal patients with hormone-dependent advanced breast cancer: a prospective, randomized, phase III study. Am J Clin Oncol. 2003;26(3):317–22.
Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol. 2001;19(10):2596–606. https://doi.org/10.1200/jco.2001.19.10.2596.
Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol. 2003;21(11):2101–9. https://doi.org/10.1200/jco.2003.04.194.
Ellis IO, Bartlett J, Dowsett M, Humphreys S, Jasani B, Miller K, et al. Updated recommendations for HER2 testing in the UK. J Clin Pathol. 2004;57:233–7. https://doi.org/10.1136/jcp.2003.007724.
Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Methodol. 2012;12:9. https://doi.org/10.1186/1471-2288-12-9.
Ouwens MJ, Philips Z, Jansen JP. Network meta-analysis of parametric survival curves. Res Synth Methods. 2010;1(3–4):258–71. https://doi.org/10.1002/jrsm.25.
Fukuda T, Shirowas T, Shimozuma K, Mouri M, Dolhara H, Akabane H. Long-term Eq-5d score for patients with metastatic breast cancer; comparison of first-line oral S-1 and taxane therapies in the randomized “Select” trial. Value Health. 2015;18(7):A467.
Eyles C, Leydon GM, Hoffman CJ, Copson ER, Prescott P, Chorozoglou M, et al. Mindfulness for the self-management of fatigue, anxiety, and depression in women with metastatic breast cancer: a mixed methods feasibility study. Integr Cancer Ther. 2015;14(1):42–56. https://doi.org/10.1177/1534735414546567.
Department of Health. NHS reference costs 2015–2016. 2016. http://www.dhgovuk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_122803. Accessed 1 Oct 2018.
Curtis L. Unit costs of health and social care. 2016. http://www.pssruacuk/pdf/uc/uc2010/uc2010pdf. Accessed 1 Oct 2018.
Karnon J. A trial-based cost-effectiveness analysis of letrozole followed by tamoxifen versus tamoxifen followed by letrozole for postmenopausal advanced breast cancer. Ann Oncol. 2003;14(11):1629–33. https://doi.org/10.1093/annonc/mdg447.
National Institute for Health and Care Excellence. Fulvestrant for untreated locally advanced or metastatic oestrogen-receptor positive breast cancer [TA503]. 2018. https://www.nice.org.uk/guidance/ta503. Accessed 1 Oct 2018.
Acknowledgements
We are very grateful to the clinical experts who provided us with information during the appraisal and commented on the draft Evidence Review Group report. We also thank Karen Welch, Information Scientist, Southampton Health Technology Assessments Centre, for appraising the literature search strategies in the company’s submission, running updates of the company’s clinical effectiveness searches and searching for ongoing studies; and Jonathan Shepherd for providing feedback on the draft Evidence Review Group report.
Author information
Authors and Affiliations
Contributions
All authors have commented on the submitted manuscript and have given their approval for the full version to be published. Neelam Kalita, Keith Cooper and Olu Onyimadu summarised and critiqued the economic analysis submitted by the company. Petra Harris, Wendy Gaisford and Joanna Picot summarised and critiqued the clinical effectiveness evidence submitted by the company. Neelam Kalita and Wendy Gaisford drafted some parts of this manuscript, which were then edited and added to by Joanna Picot who completed the manuscript and responded to feedback from all other authors. All authors reviewed, critiqued and approved this manuscript. This summary has not been externally reviewed by Pharmacoeconomics.
Corresponding author
Ethics declarations
Funding
This project was funded by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Program (project number 16/54/04 STA) [see the NIHR Journals Library website for further information, https://www.journalslibrary.nihr.ac.uk/#/]. The views and opinions expressed are the authors’ and do not necessarily reflect those of the HTA Programme, National Institute for Health and Care Excellence (NICE), NIHR, National Health Service or the Department of Health. Any errors are the responsibility of the authors. This summary of the Evidence Review Group report was compiled after NICE issued the Final Appraisal Determination.
Conflict of interest
Joanna Picot, Neelam Kalita, Wendy Gaisford, Petra Harris, Oluchukwu Onyimadu and Keith Cooper have no conflicts of interest that are directly relevant to the contents of this article.
Rights and permissions
About this article
Cite this article
Picot, J., Kalita, N., Gaisford, W. et al. Fulvestrant for Untreated Hormone-Receptor Positive Locally Advanced or Metastatic Breast Cancer: An Evidence Review Group Perspective of a NICE Single Technology Appraisal. PharmacoEconomics 37, 753–762 (2019). https://doi.org/10.1007/s40273-018-0725-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-018-0725-3