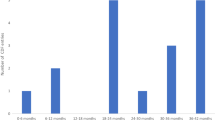
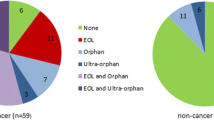
Abstract
In 2010, the Australian Government introduced the managed entry scheme (MES) to improve patient access to subsidised drugs on the Pharmaceutical Benefits Scheme and enhance the quality of evidence provided to decision makers. The aim of this paper was to critically review the Australian MES experience. We performed a comprehensive review of publicly available Pharmaceutical Benefits Advisory Committee online documents from January 2010 to July 2017. Relevant information on each MES agreement was systematically extracted, including its rationale, the conditions that guided its implementation and its policy outcomes. We identified 11 drugs where an MES was considered. Most of the identified drugs (75%) were antineoplastic agents and the main uncertainty was the overall survival benefit. More than half of the MES proposals were made by sponsors and most of the schemes were considered after previous rejected/deferred submissions for reimbursement. An MES was not established in 8 of 11 drugs (73%) despite the high evidence uncertainty. Nevertheless, six of these eight drugs were listed after the sponsors reduced their prices. Three MESs were established and implemented by Deeds of Agreement. The three cases were concluded and the required data were submitted within the agreed time frames. The need for feasibility and value of an MES should be carefully considered by stakeholders before embarking on such an agreement. It is essential to engage major stakeholders, including patient representatives, in this process. The conditions governing MESs should be clear, transparent and balanced to address the expectations of various stakeholders.
Similar content being viewed by others
References
Walker S, et al. Coverage with evidence development, only in research, risk sharing, or patient access scheme? A framework for coverage decisions. Value Health. 2012;15(3):570–9.
Claxton K, et al. A comprehensive algorithm for approval of health technologies with, without, or only in research: the key principles for informing coverage decisions. Value Health. 2016;19(6):885–91.
Grutters JP, et al. Healthy decisions: towards uncertainty tolerance in healthcare policy. Pharmacoeconomics. 2015;33(1):1–4.
Stafinski T, McCabe CJ, Menon D. Funding the unfundable: mechanisms for managing uncertainty in decisions on the introduction of new and innovative technologies into healthcare systems. Pharmacoeconomics. 2010;28(2):113–42.
Carlson JJ, Chen S, Garrison LP Jr. performance-based risk-sharing arrangements: an updated international review. Pharmacoeconomics. 2017;35(10):1063–72.
Carlson JJ, et al. Linking payment to health outcomes: a taxonomy and examination of performance-based reimbursement schemes between healthcare payers and manufacturers. Health Policy. 2010;96(3):179–90.
Australian Government Department of Health and Ageing. Memorandum of understanding between the Commonwealth of Australia and Medicines Australia. 1/10/2017. Available at: http://www.pbs.gov.au/info/news/2010/06/memorandum-of-understanding.
Australian Government Department of Health and Ageing. Draft Framework for the Managed Access Programme for submissions to the PBAC. Available at: http://www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/pbac-outcomes/2015-03/march-2015-other-matters-managed-access-programme-framewk.
Australian Government Department of Health and Ageing. Framework for the introduction of a Managed Entry Scheme for submissions to the Pharmaceutical Benefits Advisory Committee. 7/2/2018. Available at: http://www.pbs.gov.au/info/publication/factsheets/shared/framework-for-introduction-of-managed-entry-scheme-for-PBAC-submissions.
Australian Government Department of Health and Ageing. Guidelines for Deeds of Agreement. 7/2/2018. Available at: http://www.pbs.gov.au/info/industry/listing/elements/deeds-agreement.
Vitry A, Roughead E. Managed entry agreements for pharmaceuticals in Australia. Health Policy. 2014;117(3):345–52.
Wonder M, Backhouse ME, Sullivan SD. Australian managed entry scheme: a new manageable process for the reimbursement of new medicines? Value Health. 2012;15(3):586–90.
Lu CY, et al. Patient access schemes in Asia-pacific markets: current experience and future potential. J Pharm Policy Pract. 2015;8(1):6.
Pharmaceutical Benefits Advisory Committee Public summary document. Multicomponent meningococcal group b vaccine (4cmenb); 0.5 mL suspension for injection pre-filled syringe; Bexsero®. 1/10/2017. Available at: http://www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/psd/public-summary-documents-by-product#M.
Pharmaceutical Benefits Advisory Committee Public summary document. Eculizumab, 300 mg/30 mL injection, 1 × 30 mL vial injection, Soliris®. 1/10/2017. Available at: http://www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/psd/public-summary-documents-by-product#E.
Pharmaceutical Benefits Advisory Committee Public summary document. Crizotinib: Capsule 200 mg, Capsule 250 mg; Xalkori®. 1/10/2017. Available at: http://www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/psd/public-summary-documents-by-product#C.
Pharmaceutical Benefits Advisory Committee Public summary document. Trametinib: 2 mg tablet, 90, 0.5 mg tablet, 90, Mekinist®. 1/10/2017. Available at: http://www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/psd/public-summary-documents-by-product#T.
Pharmaceutical Benefits Advisory Committee Public summary document. Pembrolizumab; 50 mg injection: powder for, 1 vial, 100 mg injection: powder for, 1 vial; Keytruda®. Available at: http://www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/psd/public-summary-documents-by-product#P.
Bishop D, Lexchin J. Politics and its intersection with coverage with evidence development: a qualitative analysis from expert interviews. BMC Health Serv Res. 2013;13:88.
Hutton J, Trueman P, Henshall C. Coverage with evidence development: an examination of conceptual and policy issues. Int J Technol Assess Health Care. 2007;23(4):425–32.
Trueman P, Grainger DL, Downs KE. Coverage with evidence development: applications and issues. Int J Technol Assess Health Care. 2010;26(1):79–85.
Ferrario A, et al. The implementation of managed entry agreements in central and eastern europe: findings and implications. Pharmacoeconomics. 2017;35(12):1271–85.
Grimm SE, Strong M, Brennan A, Wailoo AJ. The HTA risk analysis chart: visualising the need for and potential value of managed entry agreements in health technology assessment. Pharmacoeconomics. 2017;35(12):1287–96.
Claxton K, Posnett J. An economic approach to clinical trial design and research priority-setting. Health Econ. 1996;5(6):513–24.
Tuffaha HW, Gordon LG, Scuffham PA. Value of information analysis in healthcare: a review of principles and applications. J Med Econ. 2014;17(6):377–83.
Bindels J, et al. Use of value of information in healthcare decision making: exploring multiple perspectives. PharmacoEconomics. 2016;34(3):315–22.
Canadian Agency for Drugs and Technologies in Health. Guidelines for the Economic Evaluation of Health Technologies: Canada. 10/10/2017. Available at: https://www.cadth.ca/about-cadth/how-we-do-it/methods-and-guidelines/guidelines-for-the-economic-evaluation-of-health-technologies-canada.
Vitry A, et al. Regulatory withdrawal of medicines marketed with uncertain benefits: the bevacizumab case study. J Pharm Policy Pract. 2015;8:25.
Edlin R, et al. Sharing risk between payer and provider by leasing health technologies: an affordable and effective reimbursement strategy for innovative technologies? Value Health. 2014;17(4):438–44.
Pharmaceutical Benefits Advisory Committee Public summary document. Imatinib, tablet, 100 mg and 400 mg (as mesylate), Glivec®. 1/10/2017. Available at: http://www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/psd/public-summary-documents-by-product#I.
Pharmaceutical Benefits Advisory Committee Public summary document. Pazopanib, tablet, 200 mg and 400 mg (as hydrochloride), Votrient®. 1/10/2017. Available at: http://www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/psd/public-summary-documents-by-product#P.
Pharmaceutical Benefits Advisory Committee Public summary document. Everolimus, tablets, 2.5 mg, 5 mg and 10 mg, Afinitor® (in SEGA). 1/10/2017. Available at: http://www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/psd/public-summary-documents-by-product#E.
Pharmaceutical Benefits Advisory Committee Public summary document. Lisdexamfetamine Dimesilate, capsules, 30 mg, 50 mg & 70 mg, Vyvanse®. 1/10/2017. Available at: http://www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/psd/public-summary-documents-by-product#JKL.
Pharmaceutical Benefits Advisory Committee Public summary document. Rifaximin, tablet, 550 mg, Xifaxan®. 1/10/2017. Available at: http://www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/psd/public-summary-documents-by-product#R.
Pharmaceutical Benefits Advisory Committee Public summary document. Blinatumomab: Injection 38.5 microgram [1 vial] and inert substance solution [10 mL vial]; Blincyto®. 1/10/2017. Available at: http://www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/psd/public-summary-documents-by-product#B.
Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of Ipilimumab in unresectable or metastatic melanoma. J. Clin. Oncol. 2015;33(17):1889–94.
Tuffaha HW, Andronis L, Scuffham PA. Setting medical research future fund priorities: assessing the value of research. Med J Aust. 2017;206(2):63–5.
Acknowledgements
Haitham Tuffaha is supported by an Australian NHMRC fellowship (GNT1121232). The authors thank Elizabeth de Somer from Medicines Australia for her contribution to the manuscript.
Author information
Authors and Affiliations
Contributions
HWT and PAS contributed to the conception and planning of the manuscript. HWT extracted and analysed the data and drafted the manuscript. PAS contributed to data interpretation, critically reviewed the manuscript and approved the final submitted version. HWT is the guarantor for the overall content of this paper.
Corresponding author
Ethics declarations
Conflict of interest
Haitham Tuffaha and Paul Scuffham declare that they have no conflicts of interest.
Research involving human participants and/or animals
Not applicable.
Informed consent
Not applicable.
Rights and permissions
About this article
Cite this article
Tuffaha, H.W., Scuffham, P.A. The Australian Managed Entry Scheme: Are We Getting it Right?. PharmacoEconomics 36, 555–565 (2018). https://doi.org/10.1007/s40273-018-0633-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-018-0633-6