Abstract
Background
Idiopathic pulmonary fibrosis (IPF) is a debilitating condition with significant morbidity and poor survival. Since 2010, there has been increased activity in the development of treatments that aim to delay progression of the disease.
Objective
Our study involves a comprehensive review of the literature for evidence on health-related quality of life (HRQoL), healthcare resource use (HCRU) and costs, and an assessment of the burden of illness of the condition.
Methods
We carried out a systematic literature review (SLR) to identify economic evaluations and HRQoL studies. We searched EMBASE, MEDLINE and MEDLINE In Process for relevant studies from database origins to April 2017. Alongside the presentation of the study characteristics and the available evidence, we carried out a qualitative comparison using reference population estimates for HRQoL and national health expenditure for costs.
Results
Our search identified a total of 3241 records. After removing duplicates and not relevant articles, we analysed 124 publications referring to 88 studies published between 2000 and 2017. Sixty studies were HRQoL and 28 were studies on costs or HCRU. We observed an exponential growth of publications in the last 3–5 years, with the majority of the studies conducted in Europe and North America. Among the HRQoL studies, and despite regional differences, there was some agreement between estimates on the absolute and relative level of HRQoL for patients with IPF compared with the general population. Regarding costs, after adjustments for the cost years and currency, the suggested annual per capita cost of patients with IPF in North America was estimated around US$20,000, 2.5–3.5 times higher than the national healthcare expenditure. Additionally, studies that analysed patients with IPF alongside a matched control cohort suggested a significant increase in resource use and cost.
Conclusion
The reviewed evidence indicates that IPF has considerable impact on HRQoL, relative to the general population levels. Furthermore, in studies of cost and resource use, most estimates of the burden were consistent in suggesting an excess cost for patients with IPF compared with a control cohort or the national health expenditure. This confirms IPF as a growing threat for public health worldwide, with considerable impact to the patients and healthcare providers.
Similar content being viewed by others
Acquiring knowledge on the overall burden of idiopathic pulmonary fibrosis (IPF) is essential for stakeholders planning resource allocation across many conditions. This study provides an overview of the evidence on health-related quality of life (HRQoL) and costs in IPF. |
Several studies showed that IPF has a considerable impact on patients’ HRQoL, including physical and social components, in comparison with the general population. |
Compared with the national health expenditure or control-matched patient cohorts, IPF was associated with an excess healthcare cost. |
Our findings confirm IPF as a growing threat for public health worldwide, with considerable impact to the patients and healthcare providers. |
1 Introduction
Idiopathic pulmonary fibrosis (IPF) is a specific form of chronic, progressive fibrosing interstitial pneumonia of unknown aetiology associated with significant morbidity and poor survival [1]. The symptoms include dyspnoea, dry cough, tiredness, aching of muscles and joints, unintended weight loss and finger clubbing [1]. The progression of the disease varies significantly between patients and depends on many clinical and external factors [2]. Overall, individuals with IPF have similar life expectancy to those with non-small cell lung cancer, with reported estimates of median survival being 50% at 3 years and 20% at 5 years post-diagnosis [1, 3,4,5]. The estimates of incidence and prevalence of IPF vary depending on the definition used, the study design, and the underlying population characteristics (such as age, gender, geographic location, etc.) [3, 6]. In general, studies agree that the condition is more common in men and in older people. In Europe, the British Thoracic Society estimates that the prevalence is around 50 per 100,000 population, with the highest rates in Northern Ireland, North West England, Scotland and Wales [7]. This is considerably higher than older estimates from other parts of Europe such as Norway (19.7–23.9/100,000) [8] and Belgium (1.25/100,000) [9]. In North America, two US studies placed the prevalence estimates between 42.7 [10] and 63 [11] patients per 100,000 population (using the broad definition); while a more recent Canadian study reported the prevalence to be as high as 115/100,000 (broad definition) [12]. Similarly, in Japan studies suggested prevalence estimates from 2.9/100,000 in 2005 [13] to 10/100,000 population in 2007 [4]. It follows that, although IPF is still treated as a rare condition in many countries, the evolution of diagnostic methods and greater physician awareness around the disease and an aging population may be leading to an increase in the prevalence and incidence rates over time [6, 14, 15].
There is also considerable activity in the development of treatments for the condition. Before 2010 there was no licensed pharmacological treatment for this devastating disease [1]. In 2008, pirfenidone was approved in Japan and in 2011 by the European Medicines Agency (EMA). In 2014 the US Food and Drug Administration (FDA) approved both pirfenidone and nintedanib, with EMA also confirming approval for nintedanib soon after [16,17,18].Footnote 1 Despite the recent termination of the clinical trial programmes for tralokinumab [19] and simtuzumab [20], a number of new agents are being tested in experimental trials for the treatment of IPF (SAR156597 [21], lebrikizumab [22], FG-3019 [23], PRM-151 [24] and others).
For healthcare providers, who often have to make difficult decisions about resource allocation across many conditions, in-depth knowledge of the overall burden of the disease is essential. Our study involves a comprehensive review of the literature for evidence on health-related quality of life (HRQoL) and costs. It also attempts a qualitative comparison with estimates of HRQoL for the general population and national healthcare expenditure to illustrate the burden of illness of IPF.
2 Methods
The study followed the PRISMA (Preferred Reporting Items for Systematic review and Meta-Analysis) guidelines.
2.1 Search Strategy
Two separate systematic reviews were conducted for economic evaluations and HRQoL evidence. Using the Ovid interface, the databases EMBASE, MEDLINE and MEDLINE In Process were searched for relevant studies. Search terms included disease-specific, economic or cost, and HRQoL keywords such as ‘idiopathic AND pulmonary AND fibrosis’, ‘fibrosing alveolitis’, ‘interstitial pneumonia’, ‘costs and cost analysis’ and ‘health care costs’, ‘HRQoL’, ‘EQ-5D’.Footnote 2
A review of HRQoL was conducted in August 2014 for the development of an economic analysis [25]. All the relevant records from the 2014 review were retrieved and the searches were updated from January 2014 to April 2017.
The economic data search was conducted from database origins to April 2017.
All references were imported into Endnote and duplicate citations were removed.
2.2 Study Selection
A review protocol with inclusion and exclusion criteria was developed at the outset of the study. The inclusion criteria were for adult patients with IPF without any restrictions on the therapy received. Other criteria included the reporting of unit costs, resource use, and HRQoL measures. To increase homogeneity in the study population characteristics, we excluded records that reported costs of diagnosis of interstitial lung disease (ILD).
The protocol was modified during the study to exclude abstract-only records published before 2015 (most often conference proceedings). Those records rarely provided sufficient information on methods and results that could be useful in our study and in general lack the scrutiny of full journal articles. Nevertheless, more recent records (post-2014) were included in our study, as we assumed that at the time of our search they were in development to a manuscript.
Screening of records was conducted in two phases (title/abstract and full-text). One experienced reviewer covered each dataset of records for economic evaluations and HRQoL evidence (EW and KV, respectively). A quarter of the records were screened independently by a second reviewer (AD, LC). If the decision for inclusion or exclusion was different in more than 10%, the full set of records were reviewed again. Because of a > 10% disagreement in the HRQoL dataset, all records were screened in a double-blind manner. The bibliography of another literature review study [26] was used to validate our findings.
2.3 Data Extraction and Analysis
Key pieces of information from the selected studies were extracted in piloted tables by three experienced researchers (EW, KV, LC). A quality check of the data extraction was done by AD. The tables were different for HRQoL and economic evidence. Given the heterogeneity of the economic evidence, we later separated studies that reported healthcare resource use or costs from economic evaluations (cost-effectiveness or budget impact analyses).
3 Results
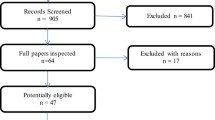
The database searches identified a total of 3241 records. After removing duplicate records, 2496 abstracts were screened against the eligibility criteria. Twelve additional records were identified via bibliography searches.
A total of 127 publications were included in the qualitative analysis, referring to 66 HRQoL and 28 economic studies. The economic studies were further categorised, with 18 reporting resource use or costs and 10 reporting on cost-effectiveness or budget impact analyses. The overall breakdown of the screening process in the reviews is presented in a PRISMA flow diagram (Fig. 1).
The studies on HRQoL increased over time with almost half conducted and published in the 3.5 years between 2014 and 2017 (see Fig. 2).Footnote 3 We did not identify any cost or economic evaluation studies conducted before 2010, while more than half of the cost studies were published in the last 3 years.
In terms of geographic regions, the majority of the studies were conducted in Europe and North America (USA and Canada) (Fig. 3). The most studied country was the USA with 13 HRQoL [27,28,29,30,31,32,33,34,35,36,37,38,39,40] and eight economic evidence publications [41,42,43,44,45,46,47,48]. From low income and lower middle income countries (using the World Bank definition [49]) we identified two studies on HRQoL from Egypt [50, 51] and one from India [52]. From east Asia the predominant country was Japan with nine HRQoL studies [53,54,55,56,57,58,59,60,61]; one study was identified from China (HRQoL) [62] and one from Korea (costs) [63]. In the HRQoL dataset, for a number of studies we did not identify a clear country of origin [64,65,66,67].
3.1 Health-Related Quality of Life Evidence
A total of 66 studies were identified (33 in the pre-2014 analysis and 33 post-2014) with HRQoL data in IPF populations. Details of the study location, the population, the HRQoL assessment tools used, and the time points, as well as the sources of funding, are presented in Table 1.
In all studies, apart from Jastrzebski et al. [69], the population mean age was over 50 years old, with the average age around 65–70 years old. The study populations were predominantly male with the exception of three studies reporting a higher proportion of female [32, 51] or an equal male/female ratio [30].
The majority of the studies used the disease-specific HRQoL instrument, St. George’s Respiratory Questionnaire (SGRQ), reported in 41 studies. Most of the studies measuring HRQoL with the SGRQ reported results for the three categories: symptoms, impact and activity; in addition to the total score. Despite the development and validation of an IPF-specific version of the SGRQ, the SGRQ-I [70], most investigators, apart from Gaunaurd et al. [28, 71, 72], continue to use the original version.
In addition, six studies reported other disease-specific HRQoL scores such as A Tool to Assess Quality of life in IPF (ATAQ-IPF) [37] or the King’s Brief Interstitial Lung Disease (K-BILD) [73]. The 36-Item Short Form Survey (SF-36) was reported in 26 studies, the EuroQol 5-level questionnaire (EQ-5D) in four studies [39, 40, 67, 74, 75], the SF-12 in two studies and one Canadian study reported Health Utilities Index Mark 2 (HUI2) scores. One study was assessing the mapping of SGRQ data to EQ-5D [76] and another study provided a mapping algorithm from SGRQ data to SF-36 [77]. Further, EQ-5D estimates from phase III trials with nintedanib in IPF (INPULSIS® I and II) were available from an economic evaluation identified during the economic data search [25].
Table 2 reports on a subsection of the studies we found that included HRQoL values based on multi-attribute preference-based measures (EQ-5D and HUI2). We obtained population reference scores for EQ VAS and EQ-5D from a survey conducted across 24 countries [78]. The survey presented scores by age and we selected the 65–74-year age category as the most representative of the IPF studies that we are using in our comparison. To obtain a reference for HUI2 scores, we looked at the US National Health Measurement Study (NHMS) using the scores for ages 65–74 years [79].
Overall, the HRQoL was found to be lower for patients with IPF compared with the general population (Fig. 4). In the German registry, INSIGHTS-IPF, the EQ VAS of the patients with IPF, was about 9 points lower on the scale compared with the population reference data [80,81,82,83,84]. The difference in the EQ-5D index score was 0.223 lower than the reference. The incremental difference between patients with IPF and the population reference is smaller in the US study STEP-IPF: around 7.5 points on EQ VAS and around 0.1 on EQ-5D index scores [67]. Furthermore, in the study by Rinciog et al. [25], the reported difference in EQ-5D index score ranges from a category with relatively good lung function (forced vital capacity [FVC] > 90% predicted: 0.84) to very poor (FVC < 50% predicted: 0.67).
EuroQol 5-level questionnaire (EQ-5D) in patients with idiopathic pulmonary fibrosis (IPF) compared with the general population (reference). FVC forced vital capacity. Asterisk indicates data from by Rinciog et al. were available by FVC% predicted status. The lowest and highest of the available intervals are shown in the figure [25]
On the HUI2 instrument, the IPF population utility estimates were substantially lower than those measured on the EQ-5D scale, both for the first year with IPF (0.585) and the fourth year (0.432) [12]. However, some of the difference with the reference scores may be attributed to country variations (US data were used for HUI2 reference).
Regarding other multi-attribute instruments, eight studies reported the average score or the mental and physical component scores (MCS and PCS) of SF-36 [27, 29, 34, 35, 39, 40, 67, 69, 85, 86]. One study reported an SF-36 score of 32 ± 11.4 for severe IPF (defined as diffusing capacity of the lungs for carbon monoxide [DLCO] < 30%) and 59.1 ± 17.8 for patients with mild-to-moderate IPF (DLCO > 30%) [27]. King et al. reported the SF-36 score of 45.7 for placebo and 45.2 for people treated with bonsentan [86]. At baseline, SF-36 PCS scores varied between 26.0 ± 8.0 [85] to 40.6 ± 9.3 [40], with an average value of 35 and SF-36 MCS ranging from 42 [69] to 55.7 ± 7.4 [40] with an average value of 48. The 17 remaining studies detailed the SF-36 results by questionnaire items (physical functioning, social functioning, mental health, role limitations due to physical problems, role limitations due to emotional problems, vitality, bodily pain, and general health perceptions).
3.2 Cost and Healthcare Resource Use Evidence
A total of 18 studies were identified with HCRU and cost evidence (Table 3). The majority were retrospective cohort analyses of claims data. Three studies were based on a synthesis of HCRU and national costs or tariffs [87,88,89]. One study was based on randomised clinical trial evidence [90] and one study was based on clinical expert opinion [91].
The most common reported resource or cost was hospitalisation (all-cause and/or respiratory-related), emergency room visits, and acute IPF exacerbation events. The majority of the studies [14] reported costs alongside resource use. Four studies reported only HCRU data [47, 90, 92, 93].
Eight of the studies that reported costs presented estimated total cost per capita [41, 42, 44, 63, 88, 91, 94, 95] (see Table 4). In three US studies the annual total cost of IPF was estimated at around US$20,000 per patient [41, 42, 44]. Controlled for the year the studies were conducted, this estimate was around three times the national per capita health expenditure [96].
In 2012, Collard et al. [42] also presented the total costs per person-year for patients with IPF and a matched control cohort (US$26,378 vs US$14,254). In a different study, published a few years later (2015), Collard et al. [41] showed similar estimates of the difference between patients with IPF and controls (US$20,887 vs US$8932).
In a study from Canada [94], the annual cost per patient with IPF was lower than the US studies [41, 42, 44]. However, in relative terms the study estimated a > 3 times greater cost when comparing with the per capita Canadian national heath expenditure.
The annual total cost per patient in Korea [63] was estimated to be < 10% of the cost presented in the American studies [41, 42, 44]. In the same study, the contribution of hospital admission costs to the total healthcare cost was found to be 86.7–88.8%. We also found great disparity in the estimates of the two studies from Spain [91, 97].
An abstract by Hill et al. [88] conducted a bottom-up cost analysis of service provision costs (excluding treatments) in England (NHS) in 2014. They estimated that the actual cost of services was over 40% of the tariff reimbursed by the NHS for each patient with IPF.
From the studies that reported resource use, Wu et al. [93] presented evidence of HCRU in US patients with IPF compared with a matching control cohort (1:3 matching ratio). They found that the mean differences between patients with IPF and control were more pronounced in outpatient hospital visits (7.5 vs 2.7), physician office visits (16 vs 7.8), and oxygen therapies (7.8 vs 0.6). After a multivariate adjustment, the magnitude of the difference was reduced for the outpatient hospital, physician and emergency room visit statistics. Nevertheless, it remained significantly higher for patients with IPF versus non-IPF.
Only five studies reported treatment costs [42, 44, 63, 89, 91]. In Kim et al., treatment costs were between 8–10% of the total costs [63]. However, it was not reported which treatment was considered. In the remaining studies, treatments included corticosteroids, oxygen therapy, azathioprine, cyclophosphamide, N-acetylcysteine (NAC), pulmonary rehabilitation therapy and lung transplantation. Of the new treatments, in Morell et al. it was reported that pirfenidone was offered to patients with IPF on compassionate grounds; it is unclear whether the cost of pirfenidone contributed to the treatment costs in that study [91].
3.3 Economic Evaluations
Ten studies were identified assessing the cost effectiveness, or budget impact, of specific treatment interventions. Details of the methods and results of the studies are presented in Table 5. Three studies were from the UK [25, 26, 98], while the remaining were from France [99], Greece [100], Italy [101, 102], Spain [103], Mexico [104] and USA [105]. The comparators included triple therapy (azathioprine, NAC and steroids), a combination of triple therapy and a genotypic assay thiopurine S-methyltransferase (TPMT), co-trimoxazole, sildenafil, pirfenidone, nintedanib and best supportive care. Only one economic evaluation included lung transplantation as an option for patients [26].
Most studies used a model to synthesise clinical, HRQoL and cost evidence. Moreover, the majority of the analyses used the direct healthcare perspective. Wilson et al. [98] conducted an economic evaluation alongside a multi-centre, randomised, placebo-controlled, double-blind trial of 12 months duration, and reported cost-effectiveness results on both the healthcare direct medical and societal perspectives.
In the economic models, the time horizon ranged between 1, 5 and 30 years, and patient lifetime. A state transition model was used for all papers, and when reported, results were calculated by a cohort analysis. In the long time-horizon models, the cost results varied between US$4000 (£3000) for BSC, US$7000 for NAC and over US$90,000 for new treatments such as pirfenidone and nintedanib. HRQoL benefits ranged between 3 and 4 QALYs. There was a noticeable distinction in the cost effectiveness of old pharmacologic technologies such as triple therapy or NAC, with estimates between US$5000–US$70,000 per QALY, and that of new treatments that exceeded US$100,000 per QALY.
4 Discussion
This was a review of HRQoL, resource use, costs and treatment cost-effectiveness studies conducted over the last 20 years in many countries, and with a variety of objectives, sources of data, and methodologies. As such, it is difficult to express with one coherent estimate the burden of illness of IPF. Nevertheless, several trends appeared in both quality of life and costs.
As with other respiratory conditions, the impact of IPF is not only limited to a worsening of the patient’s breathing function. It has wider consequences for HRQoL including physical (body weight loss, fatigue, clubbing) and social ones (recreational activities, relationships etc.). When reviewing the HRQoL evidence, this review reported on most instruments used in the literature, but focused on generic preference-based measures (such as EQ-5D) to quantify the burden of the disease. By using EQ-5D it is possible to make a comparison between the HRQoL levels with the condition versus the general population, and a comparison across other non-respiratory diseases. Furthermore, EQ-5D is increasingly used in health economic evaluations to calculate quality-adjusted life-years (QALYs), and this work presents a comprehensive review of the available evidence.
Despite the regional differences, there was some agreement between study estimates on the absolute level of HRQoL for patients with IPF; in EQ-5D, scores varied between 0.67 (± 0.242) [67] and 0.8 (± 0.2) [106]. To put this in context, the EQ-5D of patients with arthritis/rheumatism/fibrositis was reported to be 0.597 (CI 0.584–0.609; N = 4145), with hypertension/high blood pressure 0.777 (CI 0.765–0.788; N = 3172) and with asthma 0.797 (CI 0.779–0.814; N = 2452) [107, 108]. In the studies analysed, the decrement in HRQoL for patients with IPF compared with the reference population statistics was between 0.1 and 0.2 points in the EQ-5D.
With regards to costs, three US studies produced comparable estimates of costs per patient around US$20,000 [41, 42, 44]. After adjustments for the study years and currency, the suggested annual per capita cost of IPF patients in North America was estimated between 2.5–3.5 times the national health care expenditure.
We observed discrepancy in the estimates coming from two Spanish studies. This is probably attributed to the methods used. Pedraza-Serrano et al. [97] used data from a Spanish National Hospital Database (CMBD, Conjunto Mínimo Básico de Datos) and conducted a retrospective, descriptive, epidemiological study. Morell et al. [91] took a different approach by synthesising expert opinion from 15 clinicians with unit costs from national formularies. Moreover, Morell et al. [91] included treatments costs, although treatment allocation was not reported. The two estimates are very different to values from the other countries (in absolute and relative terms), which makes it very challenging to select the most accurate. The study by Pedraza-Serrano et al. [97] follows the general trend of a higher per annum cost than the national health expenditure.
Among the cost evidence identified in the literature, we emphasised the existence of matched control cohort studies [41, 42, 93]. These papers provided a direct comparison of the excess costs and resource use of IPF patients versus a reference population. Given that these studies were large in sample size and from a contemporary (2012 and 2015) and generalisable database, they produced relevant estimates for the cost burden of illness of IPF. Therefore, we recommend the use of control or reference cohorts when conducting cost analyses as it provides the relevant benchmark for comparison with the general population.
Two studies also suggested a strong correlation between acute exacerbations of IPF and other external conditions such as seasonality. Collard et al. [41] reported that acute exacerbations of IPF become more frequent in spring and winter. Kim et al. [63] highlighted spring as the season with most events, and linked that to the yellow dust phenomena occurring during that period in Korea, where this study was conducted.
The reader should note the relevance of national guidelines and prescription rules when comparing costs from different countries. Countries with a single (public) payer system, like the UK, have different practices and prescription rules to multiple-payer systems such as Germany in Europe or the US. It is also relevant to consider that some countries may have delayed access to new treatments; for instance, Australia only gained access to new anti-fibrotic agents in 2017, while Europe and the US has had access since 2010–2015.
The evidence on treatment economic evaluations was sparser. The cross-comparison of cost-effectiveness analyses is often hindered by different methodologies, time horizons, approaches in the presentation of the results and many other factors. On this occasion, an additional challenge was that most studies were published only as conference abstracts and, as such, provided little information on their methods and results. This made any comparison or synthesis of cost-effectiveness estimates very difficult.
One omission of our cost estimates is related to the diagnosis of IPF. The diagnostic procedures are largely in common with other ILDs and in most diagnostic cost studies evidence was presented from a heterogenous cohort that included patients with IPF as a subgroup [87, 109]. To include only studies that had an IPF subgroup may have been a misrepresentation of the actual management costs. For internal consistency with our population criteria, we decided to keep the reference database specific to IPF and excluded diagnostic cost studies from our review.
Our qualitative comparison of HRQoL and cost estimates with population reference statistics has further limitations. The synthesis of evidence from various studies involved the comparison of different EQ-5D versions (3L vs 5L) and conversions of cost estimates to one currency. This required several assumptions about the comparability of the data.
This review excluded relevant conference proceedings (published only as abstracts) before 2015. Records published since 2015 were included. Although the information from an abstract is often limited and the research lacks the scrutiny of an academic journal, we considered it important to include more recent records that report relevant information and that could later be published as full manuscripts. This improves the comprehensiveness of the records presented in this review.
However, the inclusion of abstracts could bias the synthesised data used to estimate the burden of illness. For instance, in the HRQoL studies we included data from the INSIGHTS-IPF registry [80,81,82,83,84] and Fell et al. [12] that at the time were available only as abstracts. In the cost studies we included Hill et al. [88] and Mittmann et al. [94].
In our search for evidence on the burden of IPF, we identified other similar literature reviews. Loveman et al. [26] conducted a systematic review with the objective being the comparison of the clinical effectiveness and cost effectiveness of IPF treatment interventions. Our study was not searching specifically for treatment effects, although there was a lot of overlap in our searches for HRQoL and economic evaluations; we identified the same papers in HRQoL and economic evaluations as Loveman et al. In addition, we have used Loveman et al. to validate our review findings [26] within the overlapping time periods.Footnote 4
Lee et al. [6] reported on the unmet public health need with IPF. Although they cover quality of life and resource utilisation, their analysis on the burden of the disease was focused more around the epidemiology, comorbidities and symptoms of IPF.
The treatment of IPF has changed substantially in recent years, and has evolved a lot since the first paper identified in our search was published (2000). We identified an exponential growth of publications in the last 3–5 years. This trend probably follows the development of new pharmacological interventions such as pirfenidone and nintedanib. For instance, we identified many publications referring to results from three nintedanib clinical trials—TOMORROW, INPULSIS® I and II [25, 110,111,112,113,114,115,116,117,118,119,120,121].
With the exception of the evidence reported in the cost-effectiveness studies, our review did not capture the full effect of new treatments in IPF. As the pipeline of available treatments expands, new research will be added to the existing data. We recommend a timely update of this review to capture the influx of new studies and any contemporary research. This will be crucial when informing policy decisions in diagnosis, treatment and palliation of patients with IPF.
5 Conclusion
IPF is a chronic, debilitating condition affecting a growing proportion of the population; predominantly male and the elderly. Our review found evidence of an important health burden of the disease in comparison with HRQoL levels of the general population. Furthermore, our review highlighted an excess cost and resource use for healthcare providers. This confirms IPF as a growing threat for public health worldwide with considerable impact on both patients and healthcare providers.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Notes
Nintedanib was also approved in Japan, Canada, Switzerland and many other countries.
For details on the search strings see the electronic supplementary material.
Note that searches were conducted in April of 2017; hence, only one quarter of the last year contributed to our results.
Some studies included in Loveman et al., not available in the English language, were not selected in our review, given our protocol inclusion criteria.
References
Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824.
The National Institute for Health and Care Excellence. Idiopathic pulmonary fibrosis. The diagnosis and management of suspected idiopathic pulmonary fibrosis. 2013.
British Thoracic Society. The British Thoracic Society Interstitial Lung Disease Registry Programme. Annual report 2015/16. 2016.
Natsuizaka M, Chiba H, Kuronuma K, Otsuka M, Kudo K, Mori M, et al. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am J Respir Crit Care Med. 2014;190(7):773–9.
Vancheri C, Failla M, Crimi N, Raghu G. Idiopathic pulmonary fibrosis: a disease with similarities and links to cancer biology. Eur Respir J. 2010;35(3):496–504.
Lee AS, Mira-Avendano I, Ryu JH, Daniels CE. The burden of idiopathic pulmonary fibrosis: an unmet public health need. Respir Med. 2014;108(7):955–67.
Office for National Statistics. Statistical bulletin: Population Estimates for UK, England and Wales, Scotland and Northern Ireland: mid-2016. 2016. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/bulletins/annualmidyearpopulationestimates/latest.
Von Plessen C, Grinde O, Gulsvik A. Incidence and prevalence of cryptogenic fibrosing alveolitis in a Norwegian community. Respir Med. 2003;97(4):428–35.
Thomeer M, Demedts M, Vandeurzen K. Registration of interstitial lung diseases by 20 centres of respiratory medicine in Flanders. Acta clinica Belgica. 2001;56(3):163–72.
Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174(7):810–6.
Fernandez Perez ER, Daniels CE, Schroeder DR, St Sauver J, Hartman TE, Bartholmai BJ, et al. Incidence, prevalence, and clinical course of idiopathic pulmonary fibrosis: a population-based study. Chest. 2010;137(1):129–37.
Fell CD, Hopkins RB, Kolb M, Dion G, Burke N, Goeree R. Prevalence and incidence of idiopathic pulmonary fibrosis in Canada. American Journal of Respiratory and Critical Care Medicine Conference: American Thoracic Society International Conference, ATS, 2015, p. 191.
Ohno S, Nakaya T, Bando M, Sugiyama Y. Idiopathic pulmonary fibrosis–results from a Japanese nationwide epidemiological survey using individual clinical records. Respirology. 2008;13(6):926–8.
Navaratnam V, Fleming KM, West J, Smith CJ, Jenkins RG, Fogarty A, et al. The rising incidence of idiopathic pulmonary fibrosis in the U.K. Thorax. 2011;66(6):462–7.
Nalysnyk L, Cid-Ruzafa J, Rotella P, Esser D. Incidence and prevalence of idiopathic pulmonary fibrosis: review of the literature. Eur Respir Rev. 2012;21(126):355–61.
European Medicines Agency. CHMP assessment report. EMA/76777/2015. 2015.
European Medicines Agency. CHMP assessment report. EMA/CHMP/115147/2011. 2011.
US Food and Drug Administration. FDA approves Esbriet to treat idiopathic pulmonary fibrosis. 2014. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/UCM418991_F.
ClinicalTrials.gov. A Phase 2, Randomized Dose-ranging Study to Evaluate the Efficacy of Tralokinumab in Adults With Idiopathic Pulmonary Fibrosis [NCT01629667] 2017. https://www.clinicaltrials.gov/ct2/show/NCT01629667?term=Tralokinumab&rank=2.
ClinicalTrials.gov. Study to assess the efficacy and safety of simtuzumab (GS-6624) in adults with idiopathic pulmonary fibrosis (IPF) (RAINIER) [NCT01769196]. 2013.
ClinicalTrials.gov. Efficacy and safety of SAR156597 in the treatment of idiopathic pulmonary fibrosis (ESTAIR) [NCT02345070] 2015. . https://clinicaltrials.gov/ct2/show/NCT02345070?term=NCT02345070&rank=1.
ClinicalTrials.gov. A study of lebrikizumab in participants with idiopathic pulmonary fibrosis [NCT01872689] 2013. https://clinicaltrials.gov/ct2/show/NCT01872689?term=NCT01872689&rank=1.
ClinicalTrials.gov. Evaluate the safety and efficacy of FG-3019 in patients with idiopathic pulmonary fibrosis [NCT01890265] 2013. https://clinicaltrials.gov/ct2/show/NCT01890265?term=NCT01890265&rank=1.
ClinicalTrials.gov. A trial to evaluate the efficacy of PRM-151 in subjects with idiopathic pulmonary fibrosis (IPF) [NCT02550873] 2016. https://clinicaltrials.gov/ct2/show/NCT02550873?term=NCT02550873&rank=1.
Rinciog C, Watkins M, Chang S, Maher T, LeReun C, Esser D, et al. Nintedanib cost-effectiveness in idiopathic pulmonary fibrosis in the UK. Value Health. 2016;19(7):A553.
Loveman E, Copley VR, Colquitt J, Scott DA, Clegg A, Jones J, et al. The clinical effectiveness and cost-effectiveness of treatments for idiopathic pulmonary fibrosis: a systematic review and economic evaluation. [Review]. Health Technol Assess. 2015;19(20):1–336.
Bors M, Tomic R, Perlman DM, Kim HJ, Whelan TP. Cognitive function in idiopathic pulmonary fibrosis. Chronic Respir Dis. 2015;12(4):365–72.
Gaunaurd IA, Gomez-Marin OW, Ramos CF, Sol CM, Cohen MI, Cahalin LP, et al. Physical activity and quality of life improvements of patients with idiopathic pulmonary fibrosis completing a pulmonary rehabilitation program. Respir Care. 2014;59(12):1872–9.
Natalini JG, Swigris JJ, Morisset J, Elicker BM, Jones KD, Fischer A, et al. Understanding the determinants of health-related quality of life in rheumatoid arthritis-associated interstitial lung disease. Respir Med. 2017;127:1–6.
Ryerson CJ, Cayou C, Topp F, Hilling L, Camp PG, Wilcox PG, et al. Pulmonary rehabilitation improves long-term outcomes in interstitial lung disease: a prospective cohort study. Respir Med. 2014;108(1):203–10.
Sharma N, Bhatt S, Luckhardt T, De Aandrade J. Short term outcomes of pulmonary rehabilitation in idiopathic pulmonary fibrosis. Chest Conference 2014.
Yount SE, Beaumont JL, Chen SY, Kaiser K, Wortman K, Van Brunt DL, et al. Health-related quality of life in patients with idiopathic pulmonary fibrosis. Lung. 2016;194(2):227–34.
Han M, Swigris J, Liu L, Bartholmai B, Murray S, Giardino N, et al. Gender influences health-related quality of life in IPF. Respir Med. 2010;104(5):724–30.
Lubin M, Chen H, Elicker B, Jones K, Collard H, Lee J. A comparison of health-related quality of life in idiopathic pulmonary fibrosis and chronic hypersensitivity pneumonitis. Chest. 2014;145(6):1333–8.
Raghu G, Brown KK, Costabel U, Cottin V, du Bois RM, Lasky JA, et al. Treatment of idiopathic pulmonary fibrosis with etanercept: an exploratory, placebo-controlled trial. Am J Respir Crit Care Med. 2008;178(9):948–55.
Swigris J, Fairclough D, Morrison M, Make B, Kozora E, Brown K, et al. Benefits of pulmonary rehabilitation in idiopathic pulmonary fibrosis. Respir Care. 2011;56(6):783–9.
Swigris J, Wilson S, Green K, Sprunger D, Brown K, Wamboldt F. Development of the ATAQ-IPF: a tool to assess quality of life in IPF. Health Qual Life Outcomes. 2010;8:77.
Horton MR, Santopietro V, Mathew L, Horton KM, Polito AJ, Liu MC, et al. Thalidomide for the treatment of cough in idiopathic pulmonary fibrosis: a randomized trial. Ann Intern Med. 2012;157(6):398–406.
Martinez FJ, de Andrade JA, Anstrom KJ, King TE Jr, Raghu G, Idiopathic Pulmonary Fibrosis Clinical Research Network. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2093–101.
Raghu G, Anstrom KJ, King TE Jr, Lasky JA, Martinez FJ. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012;366(21):1968–77.
Collard HR, Chen SY, Yeh WS, Li Q, Lee YC, Wang A, et al. Health care utilization and costs of idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older. Ann Am Thorac Soc. 2015;12(7):981–7.
Collard HR, Ward AJ, Lanes S, Cortney Hayflinger D, Rosenberg DM, Hunsche E. Burden of illness in idiopathic pulmonary fibrosis. J Med Econ. 2012;15(5):829–35.
Mooney JJ, Raimundo K, Chang E, Broder MS. Hospital cost and length of stay in idiopathic pulmonary fibrosis. J Med Econ. 2017;20(5):518–24.
Raimundo K, Chang E, Broder MS, Alexander K, Zazzali J, Swigris JJ. Clinical and economic burden of idiopathic pulmonary fibrosis: a retrospective cohort study. BMC Pulm Med. 2016;16(2):05.
Sharif R, Zouk A, Kulkarni T, Acosta P, Duncan S, Luckhardt T, et al. Cost of hospitalization among patients with idiopathic pulmonary fibrosis: patterns and predictors. Chest Conference: CHEST, October 2016, United States 2016.
Yu Y, Goehring E, Nguyen-Khoa BA, Holmes J, Jones J, Evans A, et al. Comorbidity burden and healthcare resource use in patients with idiopathic pulmonary fibrosis (IPF) in the United States (US) military health system. Chest Conference: CHEST October 2014, Austin, TX United States 2014.
Yu YF, Macaulay DS, Reichmann WM, Wu EQ, Nathan SD. Association of early suspected acute exacerbations of idiopathic pulmonary fibrosis with subsequent clinical outcomes and healthcare resource utilization. Respir Med. 2015;109(12):1582–8.
Yu YF, Wu N, Chuang CC, Wang R, Pan X, Benjamin NN, et al. Patterns and economic burden of hospitalizations and exacerbations among patients diagnosed with idiopathic pulmonary fibrosis. J Manag Care Spec Pharm. 2016;22(4):414–23.
The World Bank. World Bank Country and Lending Groups. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
Morsi TS, Ghobashy S, Younis G. Quality of life and psychological disorders in Egyptian patients with chronic lung diseases: clinico-physiological correlation. Egypt J Chest Dis Tuberc. 2014;63(3):731–43.
Rifaat N, Anwar E, Ali YM, Ellabban A, Hasan A. Value of pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis. European Respiratory Journal Conference: European Respiratory Society Annual Congress. 2014, p. 44.
Mishra A, Bhattacharya P, Paul S, Paul R, Swarnakar S. An alternative therapy for idiopathic pulmonary fibrosis by doxycycline through matrix metalloproteinase inhibition. Lung India. 2011;28(3):174–9.
Furukawa T, Taniguchi H, Ando M, Kondoh Y, Kataoka K, Nishiyama O, et al. The St. George’s Respiratory Questionnaire as a prognostic factor in IPF. Respir Res. 2017;18(1):18.
Matsuda T, Taniguchi H, Ando M, Kondoh Y, Kimura T, Kataoka K, et al. COPD Assessment Test for measurement of health status in patients with idiopathic pulmonary fibrosis: a cross-sectional study. Respirology. 2017;22(4):721–7.
Tomioka H, Mamesaya N, Yamashita S, Kida Y, Kaneko M, Sakai H. Combined pulmonary fibrosis and emphysema: Effect of pulmonary rehabilitation in comparison with chronic obstructive pulmonary disease. BMJ Open Respir Res. 2016;3(1):e000099.
Nishiyama O, Kondoh Y, Kimura T, Kato K, Kataoka K, Ogawa T, et al. Effects of pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis. Respirology. 2008;13(3):394–9.
Nishiyama O, Taniguchi H, Kondoh Y, Kimura T, Kataoka K, Nishimura K, et al. Health-related quality of life does not predict mortality in idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2012;29(2):113–8.
Kozu R, Senjyu H, Jenkins S, Mukae H, Sakamoto N, Kohno S. Differences in response to pulmonary rehabilitation in idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease. Respiration. 2011;81(3):196–205.
Kozu R, Jenkins S, Senjyu H. Effect of disability level on response to pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis. Respirology. 2011;16(8):1196–202.
Tomioka H, Imanaka K, Hashimoto K, Iwasaki H. Health-related quality of life in patients with idiopathic pulmonary fibrosis-cross-sectional and longitudinal study. Intern Med. 2007;46(18):1533–42.
Nishiyama O, Taniguchi H, Kondoh Y, Kimura T, Ogawa T, Watanabe F, et al. Health-related quality of life in patients with idiopathic pulmonary fibrosis. What is the main contributing factor? Respir Med. 2005;99(4):408–14.
Peng S, Li Z, Kang J, Hou X. Cross-sectional and longitudinal construct validity of the Saint George’s Respiratory Questionnaire in patients with IPF. Respirology. 2008;13(6):871–9.
Kim SW, Myong JP, Yoon HK, Koo JW, Kwon SS, Kim YH. Health care burden and medical resource utilisation of idiopathic pulmonary fibrosis in Korea. Int J Tuberc Lung Dis. 2017;21(2):230–5.
Jarosch I, Schneeberger T, Gloeckl R, Kreuter M, Neurohr C, Prasse A, et al. Effects of a 3-week pulmonary rehabilitation program in patients with idiopathic pulmonary fibrosis-a randomized, controlled trial. Conference: European Respiratory Society Annual Congress: European Respiratory Journal, 2016.
Kramer M, Vainshelboim B, Oliveira J, Yohoshua L, Wais I, Rusanov V, et al. Pulmonary rehabilitation improves exercise capacity and function in patients with idiopathic pulmonary fibrosis. American Thoracic Society International Conference, ATS American Journal of Respiratory and Critical Care Medicine, 2014.
Nolan CM, Kon SSC, Canavan JL, Jones SE, Maddocks M, Cullinan P, et al. Preferential lower limb muscle weakness in idiopathic pulmonary fibrosis: Effects on exercise capacity. European Respiratory Journal Conference: European Respiratory Society Annual Congress, 2014, p. 44.
Zisman D, Schwarz M, Anstrom K, Collard H, Flaherty K, Hunninghake G. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med. 2010;363(7):620–8.
Antoniou K, Nicholson A, Dimadi M, Malagari K, Latsi P, Rapti A, et al. Long-term clinical effects of interferon gamma-1b and colchicine in idiopathic pulmonary fibrosis. Eur Respir J. 2006;28(3):496–504.
Jastrzebski D, Kozielski J, Banas A, Cebula T, Gumola A, Ziora D, et al. Quality of life during one-year observation of patients with idiopathic pulmonary fibrosis awaiting lung transplantation. J Physiol Pharmacol. 2005;56(Suppl. 4):99–105.
Yorke J, Jones PW, Swigris JJ. Development and validity testing of an IPF-specific version of the St George’s Respiratory Questionnaire. Thorax. 2010;65(10):921–6.
Gaunaurd IA, Cardenas DD, Cahalin LP, Cohen MI, Ramos CF, Jackson RM, et al. Health-related quality of life of IPF patients in a pulmonary rehabilitation program. American Journal of Respiratory and Critical Care Medicine Conference: American Thoracic Society International Conference, ATS, 2014, p. 189.
Gomez O, Gaunaurd IA, Cohen M, Cardenas D, Cahalin L, Ramos C. Health-related quality of life in IPF patients on a pulmonary rehabilitation program. American Journal of Respiratory and Critical Care Medicine Conference: American Thoracic Society International Conference, ATS, 2014, p. 189.
Ferrara G, Carlson L, Palm A, Einarsson J, Olivesten C, Skold M. Idiopathic pulmonary fibrosis in Sweden: report from the first year of activity of the Swedish IPF-Registry. Eur Clin Respir J. 2016;3:310–90.
Kreuter M, Wirtz H, Prasse A, Pittrow D, Koschel D, Klotsche J, et al. Symptoms and quality of life in relation to lung function and comorbidities in patients with idiopathic pulmonary fibrosis: INSIGHTS-IPF registry. European Respiratory Journal Conference: European Respiratory Society Annual Congress, 2016, p. 48.
King TE Jr, Brown KK, Raghu G, du Bois RM, Lynch DA, Martinez F, et al. BUILD-3: a randomized, controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184(1):92–9.
Freemantle N, Wilson A, Fisher M. Mapping the St george’s respiratory questionnaire to the Euroqol 5 dimensions: a study in patients with idiopathic pulmonary fibrosis. Value Health. 2015;18(7):A503.
Swigris JJ, Brown KK, Behr J, du Bois RM, King TE, Raghu G, et al. The SF-36 and SGRQ: validity and first look at minimum important differences in IPF. Respir Med. 2010;104(2):296–304.
Szende A, Janssen B, Cabases J. Population norms for the EQ-5D. Self-reported population health: an international perspective based on EQ-5D. Dordrecht: Springer; 2014.
Fryback DG, Dunham NC, Palta M, Hanmer J, Buechner J, Cherepanov D, et al. US norms for six generic health-related quality-of-life indexes from the National Health Measurement study. Med Care. 2007;45(12):1162–70.
Behr J, Kreuter M, Hoeper M, Klotsche J, Wirtz H, Koschel D, et al. Late-breaking abstract: Current management of patients with idiopathic pulmonary fibrosis in clinical practice: INSIGHTS-IPF registry. European Respiratory Journal Conference: European Respiratory Society Annual Congress, 2014, p. 44.
Behr J, Kreuter M, Wirtz H, Hoeper M, Klotsche J, Koschel D, et al. Insights on the management of patients with idiopathic pulmonary fibrosis in clinical practice: Insights-IPF. American Journal of Respiratory and Critical Care Medicine Conference: American Thoracic Society International Conference, ATS, 2014, p. 189.
Behr J, Wirtz H, Pittrow D, Klotsche J, Koschel D, Andreas S, et al. Clinical course of patients with idiopathic pulmonary fibrosis under real life conditions: Outcomes data of the insights-IPF register. American Journal of Respiratory and Critical Care Medicine Conference: American Thoracic Society International Conference, ATS, 2015, p. 191.
Kreuter M, Birring SS, Wijsenbeek M, Wapenaar M, Oltmanns U, Costabel U, et al. German validation of the “King’s Brief Interstitial Lung Disease (K-Bild) Health Status Questionnaire”. [German] Deutschsprachige Validierung des “King’s Brief Interstitial Lung Disease (K-BILD)” Lebensqualitatsfragebogens fur interstitielle Lungenerkrankungen. Pneumologie. 2016;70(11):742–6.
Pittrow D, Klotsche J, Kreuter M, Hoeper MM, Wirtz H, Koschel D, et al. Symptom burden and health related quality of life in patients with idiopathic pulmonary fibrosis in clinical practice: insights-IPF registry. Value Health. 2014;17(7):A600.
Yazdani A, Singer LG, Strand V, Gelber AC, Williams L, Mittoo S. Survival and quality of life in rheumatoid arthritis-associated interstitial lung disease after lung transplantation. J Heart Lung Transplant. 2014;33(5):514–20.
King J, Behr J, Brown K, Du Bois R, Lancaster L, De Andrade J, et al. BUILD-1: a randomized placebo-controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177(1):75–81.
Goode CC, Mulgirigama A, Mathieson N. Idiopathic pulmonary fibrosis exacerbations: what is the cost of the diagnosis? CONFERENCE ABSTRACT: American Journal of Respiratory and Critical Care Medicine. Conference: American Thoracic Society International Conference, ATS 2014. San Diego: Conference Publication; 2014.
Hill C, Nasr R, Fisher MI, Maher T, Spiteri M, Allen M, et al. Estimated cost and payment by results (PBR) tariff reimbursement for idiopathic pulmonary fibrosis services across 14 specialist providers in England. CONFERENCE ABSTRACT: Thorax. Conference: British Thoracic Society Winter Meeting 2014. London: Conference Publication; 2014.
Nasr R, Kausar I, Chaudhuri N. Cost burden of n-acetylcysteine (NAC) in adult patients with idiopathic pulmonary fibrosis. CONFERENCE ABSTRACT: Thorax. Conference: British Thoracic Society Winter Meeting 2014. London: Conference Publication; 2014.
Diamantopoulos A, Schoof N, Esser D, LeReun C. Resource use in idiopathic pulmonary fibrosis: Influence of disease progression and exacerbations. CONFERENCE ABSTRACT: Value in Health. Conference: ISPOR 19th Annual European Congress, Austria, 2016.
Morell F, Esser D, Lim J, Stowasser S, Villacampa A, Nieves D, et al. Treatment patterns, resource use and costs of idiopathic pulmonary fibrosis in Spain—results of a Delphi panel. BMC Pulm Med. 2016;16(7):12.
Parfrey H, Leonard C, Gibbons MA, Armstrong E, Harris E, Frank R, et al. Healthcare utilisation by patients with idiopathic pulmonary fibrosis; observations from the UK pirfenidone named patient programme. Thorax. 2013;68:A164–5.
Wu N, Yu YF, Chuang CC, Wang R, Benjamin NN, Coultas DB. Healthcare resource utilization among patients diagnosed with idiopathic pulmonary fibrosis in the United States. J Med Econ. 2015;18(4):249–57.
Mittmann N, Hassan S, Seung SJ, Saherawala H, Hoffstein V, Bradley-Kennedy C. Examining health system and resource utilization and medical management of patients idiopathic pulmonary fibrosis in Ontario: a preliminary analysis. CONFERENCE ABSTRACT: Value in Health. Conference: ISPOR 18th Annual European Congress. Milan: Conference Publication; 2015.
Pedraza-Serrano F, De Andres AL, Jimenez-Garcia R, Jimenez-Trujillo I, Hernandez-Barrera V, Sanchez-Munoz G, et al. Emerging drugs and therapeutics for systemic sclerosis. EMBASE. 2016;21(4):421–30.
The Organisation for Economic Co-operation and Development. Health spending. https://data.oecd.org/healthres/health-spending.htm.
Pedraza-Serrano F, De Andres AL, Jimenez-Garcia R, Jimenez-Trujillo I, Hernandez-Barrera V, Sanchez-Munoz G, et al. Retrospective observational study of trends in hospital admissions for idiopathic pulmonary fibrosis in Spain (2004–2013) using administrative data. BMJ Open. 2017;7(2):e013156.
Wilson EC, Shulgina L, Cahn AP, Chilvers ER, Parfrey H, Clark AB, et al. Treating idiopathic pulmonary fibrosis with the addition of co-trimoxazole: an economic evaluation alongside a randomised controlled trial. Pharmacoeconomics. 2014;32(1):87–99.
Benard S, Schmidt A, Catella L, Porte F, Setton M, Fernandez-Montoya C, et al. Nintedanib cost-utility in idiopathic pulmonary fibrosis in France. Value Health. 2016;19(7):A556.
Tritaki G, Souliotis K, Bouros D, Papageorgiou I, Diamantopoulos A, Rinciog C, et al. Pharmacoeconomic assessment of nintedanib for the treatment of idiopa thic pulmonary fibrosis in the Greek healthcare system. CONFERENCE ABSTRACT: Value in Health. Conference: ISPOR 19th Annual European Congress, Austria, 2016.
Capano F, Filieri A, Lerda C, Bandelli GP, Buscaino A, Ielo D. Pirfenidone for treatment of idiopathic pulmonary fibrosis: cost effectiveness evaluation. Int J Clin Pharmacy. 2016;38(6):566.
Ravasio R, Ferrario M, Pata M, Thuresson P. Cost effectiveness analysis of pirfenidone for the treatment of mild to moderate idiopathic pulmonary fibrosis (IPF) compared to best supportive care and nintedanib from the Italian NHS perspective. Value Health. 2016;19(7):A556.
Soulard S, Crespo C. Cost-utility of nintedanib vs. pirfenidone in the treatment of idiopathic pulmonary fibrosis in Spain. Value Health. 2016;19(7):A555.
Pozo L, Paladio-Hernandez J. Cost-effective evaluation of pirfenidone for treating idiopathic pulmonary fibrosis in Mexico. Value Health. 2015;18(7):A840–1.
Hagaman JT, Kinder BW, Eckman MH. Thiopurine S-methyltransferase [corrected] testing in idiopathic pulmonary fibrosis: a pharmacogenetic cost-effectiveness analysis.[Erratum appears in Lung. 2011 Jun; 189(3):259]. Lung. 2010;188(2):125–32.
Pittrow D, Klotsche J, Kreuter M, Hoeper MM, Wirtz H, Koschel D, et al. Symptom burden and health related quality of life in patients with idiopathic pulmonary fibrosis in clinical practice: insights-Ipf registry. Value Health. 2014;17(7):A600.
Ara R, Brazier JE. Using health state utility values from the general population to approximate baselines in decision analytic models when condition-specific data are not available. Value Health. 2011;14(4):539–45.
Manuel DG, Schultz SE, Kopec JA. Measuring the health burden of chronic disease and injury using health adjusted life expectancy and the Health Utilities Index. J Epidemiol Community Health. 2002;56(11):843–50.
Sharp C, McCabe M, Adamali H, Medford AR. Use of transbronchial cryobiopsy in the diagnosis of interstitial lung disease—a systematic review and cost analysis. QJM. 2017;110(4):207–14.
Azuma A, Taniguchi H, Inoue Y, Kondoh Y, Ogura T, Homma S, et al. Nintedanib in Japanese patients with idiopathic pulmonary fibrosis: a subgroup analysis of the INPULSIS randomized trials. Respirology. 2017;22(4):750–7.
Costabel U, Inoue Y, Richeldi L, Collard HR, Tschoepe I, Stowasser S, et al. Efficacy of nintedanib in idiopathic pulmonary fibrosis across prespecified subgroups in INPULSIS. Am J Respir Crit Care Med. 2016;193(2):178–85.
Gohn N, Richeldi L, Cottin V, Selman M, Kimura T, Stowasser S, et al. Pooled analysis of data from the tomorrow and INPULSIS trials of nintedanib in idiopathic pulmonary fibrosis. Respirology. 2016;21:148.
Huggins J, Kaye M, Bailes Z, Esser D, Conoscenti C, Flaherty K. Efficacy and safety of nintedanib in US and Non US patients with idiopathic pulmonary fibrosis (IPF) in the INPULSIS trials. Chest Conference: CHEST. 2015;148(4 meeting abstract).
Kolb M, Kimura T, Stowasser S, Hallmann C, du Bois R. Effect of baseline FVC on decline in lung function with nintedanib in patients with IPF: results from the INPULSIS® trials. Am Thorac Soc. 2015.
Raghu G, Wells A, Nicholson AG, Richeldi L, Flaherty KR, Le Maulf F, et al. Consistent effect of nintedanib on decline in FVC in patients across subgroups based on HRCT diagnostic criteria: results from the INPULSIS trials in IPF. Thorax. 2015;70:A60–1.
Richeldi L, Brown KK, Cottin V, Selman M, Kimura T, Stowasser S. Pooled analysis of data from the tomorrow and INPULSIS trials of nintedanib in IPF. Thorax. 2015;70:A78.
Richeldi L, Costabel U, Selman M, Kim D, Hansell D, Nicholson A, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365(12):1079–87.
Richeldi L, Cottin V, du Bois RM, Selman M, Kimura T, Bailes Z, et al. Nintedanib in patients with idiopathic pulmonary fibrosis: combined evidence from the TOMORROW and INPULSIS trials. Respir Med. 2016;6(113):74–9.
Richeldi L, Du Bois R, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in patients with idiopathic pulmonary fibrosis: results of two 52-week, phase III, randomized, placebo-controlled trials (INPULSISTM). American Journal of Respiratory and Critical Care Medicine Conference: American Thoracic Society International Conference, ATS, 2014, p. 189.
Ryerson C, Kolb M, Richeldi L, Lee J, Kimura T, Stowasser S, et al. Effect of baseline gap index stage on decline in lung function with nintedanib in patients with idiopathic pulmonary fibrosis (IPF). QJM. 2016;109:S52.
Taniguchi H, Xu Z, Azuma A, Inoue Y, Li H, Fujimoto T, et al. Subgroup analysis of Asian patients in the INPULSIS trials of nintedanib in idiopathic pulmonary fibrosis. Respirology. 2016;21(8):1425–30.
Alhamad EH. Pirfenidone treatment in idiopathic pulmonary fibrosis: a Saudi experience. Ann Thorac Med. 2015;10(1):38–43.
Baddini Martinez J, Martinez T, Lovetro Galhardo F, De Castro Pereira C. Dyspnea scales as a measure of health-related quality of life in patients with idiopathic pulmonary fibrosis. Med Sci Monit. 2002;8(6):CR405–10.
Bahmer T, Kirsten AM, Waschki B, Rabe KF, Magnussen H, Kirsten D, et al. Clinical correlates of reduced physical activity in idiopathic pulmonary fibrosis. Respiration. 2016;91(6):497–502.
Crooks MG, Dudziak J, Morice AH, Hart SP. Cough in idiopathic pulmonary fibrosis: a sign of “silent” gastroesophageal reflux. American Journal of Respiratory and Critical Care Medicine Conference: American Thoracic Society International Conference, ATS, 2014, p. 189.
De Vries J, Seebregts A, Drent M. Assessing health status and quality of life in idiopathic pulmonary fibrosis: which measure should be used? Respir Med. 2000;94(3):273–8.
Dowman LM, McDonald CF, Hill CJ, Lee A, Barker K, Boote C, et al. Effect of disease aetiology on response to exercise training in patients with interstitial lung disease. American Journal of Respiratory and Critical Care Medicine Conference: American Thoracic Society International Conference, ATS, 2015, p. 191.
Dowman LM, McDonald CF, Hill CJ, Lee AL, Barker K, Boote C, et al. The evidence of benefits of exercise training in interstitial lung disease: a randomised controlled trial. Thorax. 2017;72(7):610–9.
Elfferich M, De Fries J, Drent M. Type D or ‘distressed’ personality in sarcoidosis and idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2011;28(1):65–71.
Glaspole IN, Chapman SA, Cooper WA, Ellis SJ, Goh NS, Hopkins PM, et al. Health-related quality of life in idiopathic pulmonary fibrosis: data from the Australian IPF registry. Respirology. 2017;22(5):950–6.
Jo HE, Glaspole I, Grainge C, Goh N, Hopkins PM, Moodley Y, et al. Baseline characteristics of idiopathic pulmonary fibrosis: analysis from the Australian idiopathic pulmonary fibrosis registry. Eur Respir J. 2017;49(2):pii.
Jones RM, Hilldrup S, Hope-Gill BD, Eccles R, Harrison NK. Mechanical induction of cough in idiopathic pulmonary fibrosis. Cough. 2011;7:2.
Key AL, Holt K, Hamilton A, Smith JA, Earis JE. Objective cough frequency in idiopathic pulmonary fibrosis. Cough. 2010;6:4.
Raghu G, King TE, Behr J, Brown KK, Du Bois RM, Leconte I, Roux S, Swigris J. Quality of life and dyspnoea in patients treated with bosentan for idiopathic pulmonary fibrosis (BUILD-1). Eur Respir J. 2010;35(1):118–23.
Kotecha J, Atkins C, Wilson A. Patient confidence and quality of life in idiopathic pulmonary fibrosis and sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2016;33(4):341–8.
Kozu R, Jenkins S, Senjyu H. Evaluation of activity limitation in patients with idiopathic pulmonary fibrosis grouped according to medical research council dyspnea grade. Arch Phys Med Rehabil. 2014;95(5):950–5.
Lutogniewska W, Jastrzebski D, Wyrwol J, Ksiazek B, Ochman M, Kowalski K, et al. Dyspnea and quality of life in patients referred for lung transplantation. Eur J Med Res. 2010;15(Suppl 2):76–8.
Martinez T, Pereira C, Dos Santos M, Ciconelli R, Guimaraes S, Martinez J. Evaluation of the short-form 36-item questionnaire to measure health- related quality of life in patients with idiopathic pulmonary fibrosis. Chest. 2000;117(6):1627–32.
Mermigkis C, Bouloukaki I, Antoniou K, Mermigkis D, Psathakis K, Giannarakis I, et al. CPAP therapy in patients with idiopathic pulmonary fibrosis and obstructive sleep apnea: does it offer a better quality of life and sleep? Sleep Breath. 2013;17(4):1137–43.
Mermigkis C, Bouloukaki I, Antoniou K, Papadogiannis G, Giannarakis I, Varouchakis G, et al. Obstructive sleep apnea should be treated in patients with idiopathic pulmonary fibrosis. Sleep Breath. 2014;19(1):385–91.
Nathan SD, Du Bois RM, Albera C, Bradford WZ, Costabel U, Kartashov A, et al. Validation of test performance characteristics and minimal clinically important difference of the 6-minute walk test in patients with idiopathic pulmonary fibrosis. Respir Med. 2015;109(7):914–22.
Ntolios P, Karampitsakos T, Karailidou M, Xanthoudaki M, Nena E, Kotakidou D, et al. Sleep quality among patients with idiopathic pulmonary fibrosis. European Respiratory Journal Conference: European Respiratory Society Annual Congress 2015.
Ozalevli S, Karaali H, Ilgin D, Ucan E. Effect of home-based pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis. Multidiscip Respir Med. 2010;5(1):31–7.
Raghu G, Martinez FJ, Brown KK, Costabel U, Cottin V, Wells AU, et al. CC-chemokine ligand 2 inhibition in idiopathic pulmonary fibrosis: a phase 2 trial of carlumab. Eur Respir J. 2015;46(6):1740–50.
Tzanakis N, Samiou M, Lambiri I, Antoniou K, Siafakas N, Bouros D. Evaluation of health-related quality-of-life and dyspnea scales in patients with idiopathic pulmonary fibrosis. Correlation with pulmonary function tests. Eur J Intern Med. 2005;16(2):105–12.
Tzouvelekis A, Paspaliaris V, Koliakos G, Ntolios P, Bouros E, Oikonomou A, et al. A prospective, non-randomized, no placebo-controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. J Transl Med. 2013;11:171.
Vainshelboim B, Oliveira J, Fox B, Kramer M. Effect of exercise pulmonary rehabilitation on long-term outcomes in idiopathic pulmonary fibrosis. European Respiratory Journal Conference: European Respiratory Society Annual Congress 2014.
Vainshelboim B, Oliveira J, Fox BD, Soreck Y, Fruchter O, Kramer MR. Long-term effects of a 12-week exercise training program on clinical outcomes in idiopathic pulmonary fibrosis. Lung. 2015;193(3):345–54.
Verma G, Marras T, Chowdhury N, Singer L. Health-related quality of life and 6 min walk distance in patients with idiopathic pulmonary fibrosis. Can Respir J. 2011;18(5):283–7.
Wuyts W, Bondue B, Dahlqvist C, Slabbynk H, Schlesser M, Gusbin N, et al. PROOF-registry: A prospective observational registry to describe the disease course and outcomes of IPF patients in a real-world clinical setting-An interim report. European Respiratory Journal Conference: European Respiratory Society Annual Congress 2015.
Wuyts WA, Peccatori FA, Russell AM. Patient-centred management in idiopathic pulmonary fibrosis: similar themes in three communication models. [Review]. Eur Respir Rev. 2014;23(132):231–8.
Zimmermann C, Carvalho C, Silveira K, Yamaguti W, Moderno E, Salge J, et al. Comparison of two questionnaires which measure the health-related quality of life of idiopathic pulmonary fibrosis patients. Braz J Med Biol Res. 2007;40(2):179–87.
Chen SY, Collard HR, Yeh WS, Li Q, Lee YC, Wang A, et al. An analysis of us medicare beneficiaries: burden of direct medical costs in patients with idiopathic pulmonary fibrosis. Value Health. 2014;17(7):A592.
Cottin V, Schmidt A, Catella L, Porte F, Fernandez-Montoya C, Le Lay K, et al. Idiopathic pulmonary fibrosis: hospital disease management and associated costs. Value Health. 2015;18(7):A665.
Cottin V, Schmidt A, Catella L, Porte F, Fernandez-Montoya C, Le Lay K, et al. Burden of idiopathic pulmonary fibrosis progression: a 5-year longitudinal follow-up study. PLoS ONE. 2017;12(1):e0166462.
Kim CH, Kim JY, Hwang YI, Lee CY, Choi JH, Park YB, et al. Interferon-gamma enzyme-linked immunospot assay in patients with tuberculosis and healthy adults. Tuberc Respir Dis. 2014;76(1):23–9.
Mooney J, Raimundo K, Chang E, Broder M. Mortality, costs and length of stay in patients with idiopathic pulmonary fibrosis (IPF). CONFERENCE ABSTRACT: QJM. Conference: 19th International Colloquium on Lung and Airway Fibrosis. Ireland 2016.
Navaratnam V, Fogarty AW, Glendening R, McKeever T, Hubbard RB. The increasing secondary care burden of idiopathic pulmonary fibrosis: hospital admission trends in England from 1998 to 2010. Chest. 2013;143(4):1078–84.
Raimundo K, Chang E, Broder M, Carrigan G, Zazzali J, Swigris JJ. Clinical and economic burden of idiopathic pulmonary fibrosis. American Thoracic Society International Conference, ATS 2015; Denver, United States: American Journal of Respiratory and Critical Care Medicine 2015.
Loveman E, Copley VR, Colquitt JL, Scott DA, Clegg AJ, Jones J, et al. The effectiveness and cost-effectiveness of treatments for idiopathic pulmonary fibrosis: systematic review, network meta-analysis and health economic evaluation. BMC Pharmacol Toxicol. 2014;15:63.
Acknowledgements
The authors would like to thank Laura Sawyer, Iain Fotheringham and Fiona Foster for their assistance with the screening of titles, abstracts and full texts and the quality check of the literature review. The authors are grateful to Valentina Acciai, Lizette Moros Burgos, Dirk Esser, Yianni Yu and Louise Catherine Josephine Gorham for their comments on a draft version of this manuscript.
Author information
Authors and Affiliations
Contributions
AD designed and supervised the study and wrote the manuscript with editorial and content input from NS and TM. KV, EW and AD developed the search strategy for the HRQoL and the economic and resource use review. KV, EW, and LC had a substantial contribution to screening of titles, abstracts and full texts with any discrepancies discussed with AD. KV, EW and LC performed the analysis and interpretation of data. All authors approved the final version of the report.
Corresponding author
Ethics declarations
Funding
This study was funded by Boehringer Ingelheim.
Conflict of interest
KV, EW, LC and AD are employed by Symmetron Limited, which received funding from Boehringer Ingelheim for this project. AD has in the past received funding from Boehringer Ingelheim for the contribution to original research and similar articles. TM has received consulting fees from Symmetron Limited; industry-academic research funding from GlaxoSmithKline R&D and UCB; and consultancy or speakers fees from AstraZeneca, Bayer, Biogen Idec, Boehringer Ingelheim, Cipla, Dosa, Galapagos, GlaxoSmithKline R&D, ProMetic, Roche (and previously InterMune), Sanofi-Aventis, Takeda and UCB. TM is supported by an NIHR Clinician Scientist Fellowship (NIHR Ref: CS-2013-13-017) and British Lung Foundation Chair in Respiratory Research (C17-3). NS is an employee of Boehringer Ingelheim.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Diamantopoulos, A., Wright, E., Vlahopoulou, K. et al. The Burden of Illness of Idiopathic Pulmonary Fibrosis: A Comprehensive Evidence Review. PharmacoEconomics 36, 779–807 (2018). https://doi.org/10.1007/s40273-018-0631-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-018-0631-8