Abstract
The National Institute for Health and Care Excellence (NICE) invited Servier, the company manufacturing trifluridine and tipiracil (T/T; trade name: Lonsurf®), to submit evidence for the clinical and cost effectiveness of T/T compared with best supportive care (BSC) for metastatic colorectal cancer (third-line or later). Kleijnen Systematic Reviews Ltd (KSR), in collaboration with Maastricht University Medical Center, was commissioned as the Evidence Review Group (ERG). This paper presents a summary of the company’s submission (CS), the ERG report and the development of the NICE guidance for the use of this drug in England and Wales by the appraisal committee (AC). The ERG produced a critical review of the clinical and cost effectiveness of T/T based upon the CS. In the CS, pooled evidence of two trials (a phase II trial and RECOURSE) showed that T/T resulted in a significant increase in overall survival [OS; hazard ratio (HR) 0.67, 95% CI 0.58–0.78] and progression-free survival (PFS; HR 0.46, 95% CI 0.40–0.53). The AC considered the survival benefit of T/T clinically meaningful although relatively small. The ERG highlighted that none of the participants in the phase II trial and approximately half of the RECOURSE participants (394 of 800) were from Europe, which might limit the applicability of the study findings to the NHS. Moreover, the ERG’s critical assessment of the company’s economic evaluation highlighted a number of concerns that resulted in 11 adjustments to the company’s base-case analysis. The ERG adjustments that had the largest impact were using the RECOURSE trial data only (instead of the pooled evidence), fixing errors and violations and using the utilities from the CORRECT trial (identified in the literature review) only. The ERG preferred to use the RECOURSE trial data only given the suboptimal methodology used by the company to pool the evidence. However, since there were no fundamental arguments to prevent the two trials from being pooled, the ERG also presented its base-case analysis based on the pooled effectiveness estimates. The company base-case resulted in an incremental cost effectiveness ratio (ICER) of £44,032 per QALY gained while the ERG base-case resulted in ICERs of £52,695 and £49,392 per QALY gained based on the RECOURSE trial only and pooled evidence, respectively. Since the AC concluded that the most plausible ICER was £49,392 per QALY gained, and that T/T meets end-of-life criteria, T/T was recommended as a cost effective use of NHS resources.
Similar content being viewed by others
Trifluridine in combination with tipiracil hydrochloride (T/T) was evaluated under the National Institute for Health and Care Excellence (NICE) single technology appraisal process. This process includes a review of the company’s submission on the clinical and cost effectiveness of its drug by an independent evidence review group (ERG). |
T/T statistically significantly improves progression-free survival as well as overall survival compared with best supportive care for the treatment of metastatic colorectal cancer (mCRC) in third-line or later. |
Despite the company using naïve methods to synthesise the effectiveness evidence of two trials, the pooled evidence analysis was preferred given (1) the similarity between the two trials (in design and baseline disease characteristics) and (2) that more sophisticated methods would most likely produce comparable results. |
The NICE Appraisal Committee concluded that T/T met the end-of-life criteria and that the most plausible incremental cost effectiveness ratio was £49,392 per QALY gained, and hence recommended T/T, within its marketing authorisation, as an option for treating mCRC. |
1 Introduction and the Decision Problem
The National Institute for Health and Care Excellence (NICE) recommended trifluridine in combination with tipiracil hydrochloride (T/T; trade name: Lonsurf®), within its marketing authorisation, as an option for treating metastatic colorectal cancer (mCRC). The marketing authorisation stipulates treatment for adults with mCRC who have been previously treated with, or are not considered candidates for, available therapies including fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapies, anti-vascular endothelial growth factor (VEGF) agents and anti-epidermal growth factor receptor (EGFR) agents. T/T treatment is authorised only when the company provides the discount agreed in the patient access scheme (PAS) [1].
Health technologies must be clinically effective and represent cost effective use of National Health Service (NHS) resources to be recommended by NICE for use within NHS England and Wales. T/T was appraised under the NICE single technology appraisal (STA) process which typically considers new technologies within a single indication [2]. Within the STA process, the company (Servier Laboratories Ltd) provided NICE with a written submission, including an executable health economic model detailing the company’s estimates of the clinical effectiveness and cost effectiveness of T/T. The company’s submission (CS) [3] was critically reviewed by the Evidence Review Group (ERG), Kleijnen Systematic Reviews, an external organisation independent of NICE, which produced an ERG report [4]. After consideration of the CS, the ERG report, as well as testimony from experts, patients and other stakeholders, the NICE Appraisal Committee (AC) issued the Final Appraisal Determination (FAD) [1] consisting of guidance regarding whether or not to recommend the technology, which is open to appeal.
2 Clinical Effectiveness
The CS presented a well conducted systematic review (SR) which identified two randomized controlled trials comparing T/T with best supportive care (BSC): a phase II trial (172 participants from Japan) and RECOURSE (a multinational trial with 800 participants; 394 from Europe, 9 from the UK) [5, 6]. Analyses showed that the effect of T/T did not vary according to geographical location and, as a result, the trials were pooled. The ERG agreed with the CS that both trials were of high methodological quality. Moreover, the trial data were considered mature with 89% and 73% of the patients being deceased in RECOURSE and the phase II trial, respectively.
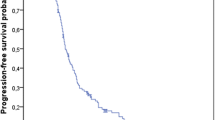
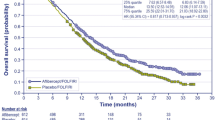
Pooled analyses indicated an increase in both median (1.9 months; T/T 7.3 months, BSC 5.4 months) and mean overall survival (OS; 2.3 months; T/T 9.1, 6.8 months) as well as both median (0.2 months; T/T 1.9 months, BSC 1.7 months) and mean progression-free survival (PFS; 1.8 months; T/T 3.7 months, BSC 1.9 months). Differences in both OS and PFS were considered statistically significant with hazard ratios (HRs) of 0.67 (95% CI 0.58–0.78) and 0.46 (95% CI 0.40–0.53), respectively. The AC considered the survival benefit of T/T to be clinically meaningful although relatively small.
For both trials combined, only one participant (that received BSC) had a complete response while nine participants (all receiving T/T) showed a partial response. The proportion of T/T patients with stable disease was greater in both trials compared with BSC (42.9 vs 10.5% in the phase II trial and 42.4 vs 15.9% in RECOURSE).
More treatment-related adverse events (AEs) were observed in the T/T arms compared with the BSC arms (RECOURSE: 85.7 vs 54.7% and phase II trial: 96.5 vs 70.2%, respectively). The number of participants experiencing serious AEs was greater for participants receiving T/T in the phase II trial (18.6 vs 8.8%), but not in the RECOURSE trial (29.6 vs 33.6%). The AC concluded that T/T has an acceptable burden of AEs.
Following a request by the ERG, the company confirmed that there is no internationally accepted definition of BSC for clinical trials, which means that the nature of BSC provided could vary between trial centres. The ERG highlighted for the AC that none of the participants in the phase II trial and approximately half of the RECOURSE participants (394 of 800) were from Europe, which might limit the applicability of the study findings to the NHS. Moreover, no results were reported for one of the NICE specified outcomes: health-related quality of life.
3 Cost Effectiveness
The company developed a de novo Excel-based partitioned-survival model to assess the cost effectiveness of T/T compared with BSC as third-line or later treatment for patients with mCRC. Health states were pre-progression, post-progression and death. These health states were selected according to the clinical pathway of care and consistent with other late-stage cancer models. The economic evaluation used the perspective of the NHS. Utilities and costs were discounted at 3.5% over a time horizon of 10 years with a daily cycle length. The company justified the time horizon of 10 years as being effectively lifetime as < 1% of patients are still alive. The company used combined data from the phase II trial [6] and the RECOURSE trial [5] to estimate OS and PFS for use in the model. PFS was also used as a proxy for time on treatment. Other parameters such as AE and T/T dosing were based on the RECOURSE trial only. The company calculated health state utility values by averaging utility values obtained from the CORRECT trial [7, 8] (identified in the SR) and the cetuximab NICE CS for the first-line treatment of mCRC [9] (i.e. TA176, not identified in the SR). The company’s base-case incremental cost effectiveness ratio (deterministic, with PAS) was £44,032 per QALY gained. Deterministic one-way sensitivity analysis, scenario analyses and probabilistic sensitivity analyses were conducted. Probabilistic sensitivity analyses indicated that at the PAS price, the probabilities of T/T being the most cost effective treatment were 0 and 77% for willingness-to-pay (WTP) thresholds of £30,000 and £50,000, respectively.
Even though pooling the effectiveness data from the RECOURSE trial and the phase II trial seemed reasonable, the methods were not clearly described in the CS. After response to a clarification question by the ERG, it appeared that individuals from both trials were naïvely combined in one dataset and compared with each other, which could generate biased treatment effect estimates. Therefore, the ERG preferred to adopt a more conservative assumption in its base-case analysis by using RECOURSE data only. However, since there were no fundamental arguments which prevent the two trials from being pooled (except usage of suboptimal methodology), the ERG also presented its base-case analysis using the pooled effectiveness estimates from both trials. Moreover, the ERG fixed an error with regards to the implementation of AE rates for the BSC arm in the company’s analyses. Finally, the ERG regarded the company’s arguments to estimate the health state utilities using an average of the utilities from TA176 [9] and the CORRECT trial [7, 8] as incorrect or based on incorrect information. According to the ERG, the baseline utilities from the CORRECT trial were the most plausible estimates, because it was the only study identified by the ERG in which utilities were measured using the European Quality of Life–5 Dimensions (EQ-5D) in a population that resembled the population in this appraisal (second- to fourth-line population with 74% third-line or greater). Therefore, the ERG included utility values from the CORRECT trial in its base-case.
Categories considered for resource use and costs were T/T costs, health-state costs, post-progression treatment costs, end-of-life costs and adverse event costs. In the company’s base-case, T/T costs were calculated based on the body surface area (BSA), treatment delay and dose reductions obtained from the RECOURSE trial. Medical resource utilisation per health state was estimated using expert opinion and included oral chemotherapy day-case attendance, medical oncologist outpatient consultation, home consultation by general practitioners (GPs), community nurse specialist visit, home health visitor, district nurse visit and GP surgery visit. This was combined with 2014–2015 NHS references costs and Personal Social Services Research Unit (PSSRU) costs from or inflated to 2015. Post-progression treatment costs were calculated based on resource use from the RECOURSE trial. Costs of adverse events that are actively treated in the NHS as well as end-of-life care costs were obtained from published literature.
Compared with the company base-case, the ICER increased by approximately £9300 to £52,695 per QALY gained in the ERG base-case (probabilistic, with PAS). This difference could largely be attributed to a reduction in incremental QALYs gained from 0.172 to 0.144. Moreover, the ERG adjustments that had the largest impact were using the RECOURSE trial data only, fixing errors and violations and using the utilities from the CORRECT trial only. The probability that T/T is cost effective was smaller in the ERG base-case compared with the company’s base-case (0% and 37% for WTP thresholds of £30,000 and £50,000, respectively). When using the pooled evidence for OS, PFS, AE, body surface area and treatment dose reductions, the ICER decreased to £49,392 per QALY gained. The AC concluded that the ERG’s ICER of £49,392 per QALY gained most closely reflected its preferred analysis [2]. The AC preferred the analysis using the ‘naively’ pooled evidence (instead of using the RECOURSE trial data only) for two main reasons, given that (1) the trials had similar designs, and patients in both trials had similar disease characteristics at baseline and (2) during the committee meeting, the company confirmed that the results were almost the same whether the evidence synthesis was performed naively or stratified (adjusted) by trial.
4 End-of-Life Criteria and Conclusion
The AC concluded that T/T meets the end-of-life (EOL) criteria. This was based on a life expectancy of 8 months (observed for BSC) and the estimated mean OS improvement of 3 months (although the ERG highlighted that the OS difference in restricted mean OS, i.e. without extrapolation, would be 2 months) [4]. Moreover, the AC indicated that in clinical practice patients may derive a greater OS benefit than in the trials because they will not have had all the therapies that the trial patients would have had. Having concluded that T/T met the EOL criteria and that the most plausible ICER was £49,392 per QALY gained, the AC recommended T/T as a cost effective use of NHS resources [2].
References
National Institute for Health and Care Excellence. Trifluridine–tipiracil for previously treated metastatic colorectal cancer. NICE technology appraisal guidance 405. NICE, London. 2016. https://www.nice.org.uk/guidance/ta405. Accessed 8 Dec 2016.
National Institute for Health and Care Excellence. Guide to the processes of technology appraisal. NICE, London. 2014. http://www.nice.org.uk/article/pmg19. Accessed 8 Dec 2016.
Servier Laboratories. Colorectal cancer (metastatic) - trifluridine with tipiracil hydrochloride, after standard therapy [ID876]. Company evidence submission to National Institute of Health and Care Excellence. Single technology appraisal (STA). Servier Laboratories; 2016.
Wolff R, Ramaekers BLT, Van Giessen A, Pouwels X, Fayter D, Lang S, et al. Trifluridine in combination with tipiracil hydrochloride for previously treated metastatic colorectal cancer: a Single Technology Appraisal. Kleijnen Systematic Reviews Ltd, York. 2016. https://www.nice.org.uk/guidance/TA405/documents/committee-papers. Accessed 8 Dec 2016.
Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372(20):1909–19.
Yoshino T, Mizunuma N, Yamazaki K, Nishina T, Komatsu Y, Baba H, et al. TAS-102 monotherapy for pretreated metastatic colorectal cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2012;13(10):993–1001.
Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–12.
Siena S, Grothey A, Sobrero A, Falcone A, Ychou M, Lenz HJ, et al. Effects of regorafenib therapy on health-related quality of life in patients with metastatic colorectal cancer in the phase III CORRECT study. Paper presented at European Cancer Congress; 27 Sep–1 Oct 2013; Amsterdam: The Netherlands. Eur J Cancer. 2013;49(27):S482.
Merck Serono Ltd. Single technology appraisal submission: erbitux (cetuximab) for the first-line treatment of metastatic colorectal cancer. Merck Serono Ltd. 2008. https://www.nice.org.uk/guidance/TA176/documents/colorectal-cancer-first-line-cetuximab-merckserono2. Accessed 11 Mar 2016.
Author information
Authors and Affiliations
Contributions
All authors have commented on the submitted manuscript and have given their approval for the final version to be published. RW, DF, SL, GW and JK reviewed the clinical effectiveness evidence, SD reviewed the search methods, BR, AG, XP, NA and MJ reviewed the cost effectiveness evidence. BR acts as overall guarantor for the manuscript. This summary has not been externally peer reviewed by PharmacoEconomics.
Corresponding author
Ethics declarations
Funding
This project was funded by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Programme. See the HTA programme website for further project information (http://www.hta.ac.uk). This summary of the ERG report was compiled after NICE issued the FAD. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of NICE or the Department of Health.
Conflicts of interest
None of the authors (BR, RW, AG, XP, DF, SL, NA, GW, SD, JK MJ) has any conflicts to declare.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ramaekers, B.L.T., Wolff, R., van Giessen, A. et al. Trifluridine–Tipiracil for Previously Treated Metastatic Colorectal Cancer: An Evidence Review Group Perspective of a NICE Single Technology Appraisal. PharmacoEconomics 36, 285–288 (2018). https://doi.org/10.1007/s40273-017-0591-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-017-0591-4