Abstract
Systematic literature reviews of health-related quality of life (HRQoL) evidence that are to inform economic models can be challenging due to the volume of hits identified in searches using generic terms for HRQoL. Nevertheless, a robust review of the literature is required to ensure that the health state utility values (HSUVs) used in the economic model are the most appropriate available. This article provides a synopsis of literature relating to identifying, reviewing and synthesising HSUVs. The process begins with scoping the needs of the economic model, including the definitions of health states and the requirements of any reimbursement agencies. A sequence of searches may be required as the economic model evolves. The terminology used for HRQoL measures may be problematic, and as there is no robust HRQoL filter [equivalent to that applied for randomised control trial (RCTs)], sifting the results of sensitive searches can be resource intensive. Alternative approaches such as forward and backward citation searches may reduce the resources required, while maintaining the integrity of the search. Any included studies should be assessed in terms of quality using a recommended checklist, and insufficient detail in the primary studies should be noted as a short-coming in this exercise. Subject to homogeneity (similar populations, same measure and preference weights) evidence can be pooled in some way, although methodological research into the appropriateness of alternative techniques for meta-analysis is in its infancy. Reporting standards are key and as a minimum should include details on searches, inclusion/exclusion criteria (together with rationale for exclusion at each stage), assessment of quality and relevance of included studies, and justification for the choice of final HSUVs.

Source: Reprinted from Papaioannou et al. [5], page 687, with permission from Elsevier

Source: Reprinted from Papaioannou et al. [5], page 689, with permission from Elsevier

Source: Adapted from Papaioannou et al. [5], page 691, with permission from Elsevier

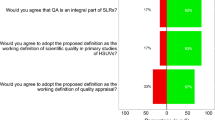
Source: Papaioannou et al. [4]
Similar content being viewed by others
References
Brazier J, Ara R, Chevrou-Severac H. W3: Utilities in economic evaluation: using best practices where international guidelines provide insufficient detail. www.ispor.org/Event/ProgramList/2016Vienna?type=Workshop. Accessed 13 April 2017.
Peasgood T, Herrmann K, Kanis JA, Brazier JE. An updated systematic review of health state utility values for osteoporosis related conditions. Osteoporos Int. 2009;20(6):853.
Kanis JA, Stevenson M, McCloskey EV, Davis S, Lloyd-Jones M. Glucocorticoid-induced osteoporosis: a systematic review and cost-utility analysis. Health Technol Assess. 2007;11(7):1–231.
Papaioannou D, Brazier JE, Paisley S. NICE DSU Technical Support Document 9: the identification, review and synthesis of health state utility values from the literature. National Health Service, 2010. http://www.nicedsu.org.uk.
Papaioannou D, Brazier J, Paisley S. Systematic searching and selection of health state utility values from the literature. Value Health. 2013;16(4):686–95.
Rowen D, Azzabi Zouraq I, Chevrou-Severac H, van Hout B. International regulations and recommendations. Current issue Pharmacoeconomics.
Guide to the methods of technology appraisal 2013 NICE. www.nice.org.uk/process/pmg9/chapter/foreword. Accessed April 2017.
Brazier J, Ara R, Rowen D, Chevrou-Severac H. A review of generic preference-based measures for use in cost-effectiveness models. PharmacoEconomics. 2017. doi:10.1007/s40273-017-0545-x.
Rowen D, Brazier J, Ara R, Azzabi Zouraq I. The role of condition-specific preference-based measures in health technology assessment. PharmacoEconomics. 2017. doi:10.1007/s40273-017-0546-9.
Ara R, Rowen D, Mukuria C. The use of mapping to estimate health state utility values. PharmacoEconomics. 2017. doi:10.1007/s40273-017-0548-7.
Critical Appraisal Skills Programme CASP (systematic review checklist). 2017. http://www.casp-uk.net/.
Arber M, Garcia S, Veale T, Edwards M, Shaw A, Glanville J. Performance of search filters to identify health state utility studies. 2016. ISPOR 19th Annual European Congress. Vienna.
Paisley S, Booth A, Mensinkai S. Health-related quality of life studies. Etext on Health Technology Assessment (HTA) information resources. Bethesda, MD: US National Library of Medicine (NLM), National Information Center on Health Services Research and Healthcare Technology (NICHSR), 2005.
Wolowacz SE, Briggs A, Belozeroff V, Clarke P, Doward L, Goeree R, Lloyd A, Norman R. Estimating health-state utility for economic models in clinical studies: an ISPOR Good Research Practices Task Force Report. Value Health. 2016;19(6):704–19.
Doth AH, Hansson PT, Jensen MP, Taylor RS. The burden of neuropathic pain: a systematic review and meta-analysis of health utilities. Pain. 2010;149(2):338–44.
Higgins J, Thompson S. Controlling the risk of spurious findings from meta-regression. Stat Med. 2004;23:1663–82.
Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to meta-analysis. New York: Wiley; 2011.
Peasgood T, Brazier J. Is meta-analysis for utility values appropriate given the potential impact different elicitation methods have on values? Pharmacoeconomics. 2015;33(11):1101–5.
Acknowledgements
The authors would like to thank Prof. Jon Karnon, PhD, of the University of Adelaide and Dr. Andrew Lloyd, PhD, of Bladen Associates Ltd for their editorial review.
Author information
Authors and Affiliations
Contributions
RA reviewed the literature, wrote the first draft and made the final edits to the manuscript. JEB made significant edits to the first and final draft of the manuscript. TP contributed to the initial draft of the manuscript and made edits to the final draft. SP made comments on the final draft of the manuscript.
Corresponding author
Ethics declarations
Disclosure statement
This article is published in a special edition journal supplement wholly funded by Takeda Pharmaceutical International AG, Zurich, Switzerland.
Funding
This study was funded by an unrestricted grant from Takeda Pharmaceuticals International AG.
Conflict of interest
Roberta Ara has no conflicts of interest. John Brazier has no conflicts of interest. Tessa Peasgood has no conflicts of interest. Suzy Paisley has no conflicts of interest.
Rights and permissions
About this article
Cite this article
Ara, R., Brazier, J., Peasgood, T. et al. The Identification, Review and Synthesis of Health State Utility Values from the Literature. PharmacoEconomics 35 (Suppl 1), 43–55 (2017). https://doi.org/10.1007/s40273-017-0547-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-017-0547-8